Abstract
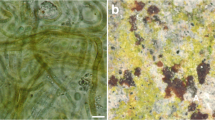
Domination of certain aerophytic phototrophic group or specific taxon in biofilms is connected with biofilm features recognised in situ. Well-developed, gelatinous, olive to dark-green biofilms are composed mostly of coccoid cyanobacterial forms. The same features, characterised biofilms dominated by one coccoid taxon, except the latter were vividly coloured. Gloeobacter caused the appearance of purple, Gloeocapsa representatives yellow and Chroococcidiopsis black biofilm. The brown to the dark colour of heterocytous biofilms was mainly caused by Nostoc. Simple trichal Cyanobacteria were occasionally present in biofilm, except in one blue-coloured sample. According to the principal component analysis (PCA), well-developed and gelatinous biofilms were correlated with Cyanobacteria, while scanning electron microscopy (SEM) revealed richness of extracellular polymeric substances (EPS) in such biofilms. Biofilm with calcified cyanobacterium (Geitleria cf. calcarea) was also found. Chlorophyta-abundant biofilms (many rich in Desmococcus), thinner than cyanobacterial, were predominantly green and occasionally yellow and blue. Many were dry when observed in situ (confirmed with PCA), with few being moistened (i.e. Klebsormidium-dominant). Diatom biofilms were usually developed on sediment, mosses or near seeping water (demonstrated by PCA) and were also thinner than cyanobacterial ones. Compared to cyanobacterial biofilms, SEM showed less developed EPS in those rich in diatoms and green algae, where microorganisms are more exposed to the environment. The study demonstrates an easy method for biofilm assessment based on visual characterisation and provides encouragement for more frequent biofilm investigation in caves that can be important from an ecological, biological, biotechnological point of view and which assessment can have an irreplaceable role in potential monitoring and protection.







Similar content being viewed by others
References
Abed, R. M. M., Dobretsov, S., & Sudesh, K. (2009). Applications of cyanobacteria in biotechnology. Journal of Applied Microbiology, 106, 1–12.
Ahmadjian, V. (1988). The lichen alga Trebouxia: does it occur free-living? Plant Systematics and Evolution, 158(2/4), 243–247.
Albertano, P. (2012). Cyanobacterial biofilms in monuments and caves. In B. A. Whitton (Ed.), Ecology of Cyanobacteria II: their diversity in space and time (pp. 317–343). Netherlands: Springer.
Ariño, X., Hernández-Mariné, M., & Saiz-Jiménez, C. (1997). Colonization of Roman tombs by calcifying cyanobacteria. Phycologia, 36(5), 366–373.
Avelar, M., Bonilla-Heredia, B., Merino-Ibarra, M., Herrera-Silveira, J. A., Ramirez, J., Rosas, H., Valdespino, J., Carricart-Ganivet, J. P., & Martínez, A. (2013). Iron, cadmium, and chromium in seagrass (Thalassia testudinum) from a coastal nature reserve in karstic Yucatán. Environmental Monitoring and Assessment, 185(9), 7591–7603.
Balskus, E. P., & Walsh, C. T. (2008). Investigating the initial steps in the biosynthesis of cyanobacterial sunscreen scytonemin. Journal of the American Chemical Society, 130(46), 15260–15261.
Balskus, E. P., Case, R. J., & Walsh, C. T. (2011). The biosynthesis of cyanobacterial sunscreen scytonemin in intertidal microbial mat communities. FEMS Microbiology Ecology, 77(2), 322–332.
Barton, H. A. (2006). Introduction to cave microbiology: a review for the non-specialists. Journal of Cave and Karst Studies, 68(2), 43–54.
Baqué, M., Vera, J. P., Rettberg, P., & Billi, D. (2013). The BOSS and BIOMEX space experiments on the EXPOSE-R2 mission: endurance of the desert cyanobacterium Chroococcidiopsis under simulated space vacuum, Martian atmosphere, UVC radiation and temperature extremes. Acta Astronautica, 91, 180–186.
Baquedano Estévez, C., Moreno Merino, L., de la Losa Román, A., & Durán Valsero, J. J. (2019). The lampenflora in show caves and its treatment: an emerging ecological problem. International Journal of Speleology, 48(3), 249–277.
Billi, D., Baqué, M., Smith, H. D., & McKay, C. P. (2013). Cyanobacteria from extreme deserts to space. Advances in Microbiology, 3, 80–86.
Borderie, F., Alaoui-Sossé, B., & Aleya, L. (2015). Heritage materials and biofouling mitigation through UV-C irradiation in show caves: stateof-the-art practices and future challenges. Environmental Science and Pollution Research, 22(6), 4144–4172.
Cennamo, P., Marzano, C., Giorgio, A., Caputo, P., & Moretti, A. (2009). Bioincrustations in the Church of Saint Maria of Piedigrotta (Pizzo Calabro, Italy). In: Proceedings of the 4th International Congress on Science and Technology for the Safeguard of Cultural Heritage in the Mediteranean Basin, Cairo, 2, 272-275.
Cennamo, P., Marzano, C., Ciniglia, C., Pinto, G., Cappelletti, P., Caputo, P., & Pollio, A. (2012). A survey of the algal flora of anthropogenic caves of Campi Flegrei (Naples, Italy) archeological district. Journal of Cave and Karst Studies, 74(3), 243–250.
Cigna, A. (2012). Show caves. In W. B. White & D. C. Culver (Eds.), Encyclopedia of caves (pp. 690–697). Amsterdam: Elsevier.
Cirés, S., Casero, M. C., & Quesada, A. (2017). Toxicity at the edge of life: a review on cyanobacterial toxins from extreme environments. Marine Drugs, 15(7), 233.
Czerwik-Marcinkowska, J., Wojciechowska, A., & Massalski, A. (2015). Biodiversity of limestone caves: aggregations of aerophytic algae and cyanobacteria in relation to site factors. Polish Journal of Ecology, 63(4), 481–499.
Davey, M. E., & O’Toole, G. A. (2000). Microbial biofilms: from ecology to molecular genetics. Microbiology and Molecular Biology Reviews, 64(4), 847–867.
De los Rios, A., Ascaso, C., Wierzchos, J., Fernández-Valiente, E., & Quesada, A. (2004). Microstructural characterization of cyanobacterial mats from the McMurdo Ice Shelf, Antarctica. Applied and Environmental Microbiology, 70(1), 569–580.
Donlan, R. M. (2002). Biofilms: microbial life on surfaces. Emerging Infectious Diseases Journal, 8(9), 881–890.
Falasco, E., Ector, L., Isaia, M., Wetzel, C. E., Hoffmann, L., & Bona, F. (2014). Diatom flora in subterranean ecosystems: a review. International Journal of Speleology, 43(3), 231–251.
Friedmann, I. (1955). Geitlerea calcarea n. gen. et n. sp. A new atmophytic limeincrusting blue-green alga. Botaniska Notiser, 108, 439–445.
Gaylarde, P. M., & Gaylarde, C. C. (1998). A rapid method for the detection of algae and cyanobacteria on the external surfaces of buildings. In C. C. Gaylarde, T. C. P. Barbosa, & N. H. Gabilan (Eds.), Third Latin American Biodegradation and Biodeterioration Symposium (p. 37). UK: The British Phycological Society.
Gorbushina, A. A. (2007). Life on the rocks. Environmental Microbiology, 9(7), 1613–1631.
Grilli-Cailola, M., & Billi, D. (2007). Chroococcidiopsis from desert to Mars. In J. Seckbach (Ed.), Algae and cyanobacteria in extreme environments (pp. 555–570). Dordrech: Springer.
Hall-Stoodley, L., Costerton, J. W., & Stoodley, P. (2004). Bacterial biofilms: from the natural environment to infectious diseases. Nature Reviews Microbiology, 2(2), 95–108.
Hernández-Mariné, M., & Canals, T. (1994). Herpyzonema pulverulentum (Mastigocladaceae), a new cavernicolous atmophytic and limeincrusted cyanophyte. Archiv für Hydrobiology Algological Studies, 75, 123–136.
Hernández-Mariné, M., Clavero, E., & Roldán, M. (2004). Microscopy methods applied to research on cyanobacteria. Limnetica, 23(1–2), 179–186.
Hoffmann, L. (2002). Caves and other low-light environments: aerophytic photoautotrophic microorganisms. In G. Bitton (Ed.), Encyclopedia of environmental microbiology (pp. 835–843). New York: Wiley.
Keshari, N., & Adhikary, S. P. (2014). Diversity of cyanobacteria on stone monuments and building facades of India and their phylogenetic analysis. International Biodeterioration & Biodegradation, 90, 45–51.
Kharkongor, D., & Ramanujam, P. (2017). Antioxidant activities of four dominant species of Trentepohlia (Trentepohliales, Chlorophyta). International Journal of Complementary and Alternative Medicine, 8(5), 00270.
Komárek, J., & Anagnostidis, K. (1998). Cyanoprokaryota. 1. Teil: Chroococcales. In H. Ettl, G. Gärtner, H. Heynig, & D. Mollenhauer (Eds.), Süβwasserflora von Mitteleuropa (p. 548). Heidelberg: Spektrum Akademischer Verlag.
Korber, D. R., James, G. A., & Costerton, J. W. (1994). Evaluation of fleroxacin activity against established Pseudomonas fluorecens biofilms. Applied and Environmental Microbiology, 60(5), 1663–1669.
Krammer, K., & Lange-Bertalot, H. (1986). Bacillariophyceae. 1. Teil: Naviculaceae. In H. Ettl, J. Gerloff, H. Heynig, & D. Mollenhauer (Eds.), Süßwasserflora von Mitteleuropa (2/1 ed., p. 876). Jena: G. Fischer Verlag.
Krammer, K., & Lange-Bertalot, H. (1988). Bacillariophyceae. 2. Teil. Bacillariaceae, Epithemiaceae, Surirellaceae. In H. Ettl, J. Gerloff, H. Heynig, & D. Mollenhauer (Eds.), Süßwasserflora von Mitteleuropa (2/2 ed., p. 596). Jena: G. Fischer Verlag.
Krammer, K., & Lange-Bertalot, H. (2004). Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In H. Ettl, J. Gerloff, H. Heynig, & D. Mollenhauer (Eds.), Süßwasserflora von Mitteleuropa (2/3 ed., p. 598). München: Elsevier GmbH.
Lamprinou, V., Hernández-Marine, M., Canals, T., Kormas, K., Economou-Amilli, A., & Pantazidou, A. (2011). Morphology and molecular evaluation of Iphinoe spelaeobios gen. nov., sp. nov. and Loriellopsis cavernicola gen. nov., sp. nov., two stigonematalean cyanobacteria from Greek and Spanish caves. International Journal of Systematic and Evolutionary Microbiology, 61, 2907–2915.
Lamprinou, V., Danielidis, D. B., Economou-Amilli, A., & Pantazidou, A. (2012). Distribution survey of Cyanobacteria in three Greek caves of Peloponnese. International Journal of Speleology, 41(2), 267–272.
Lange-Bertalot, H. (2001). Navicula sensu stricto. 10 genera separated from Navicula sensu lato. Frustulia. In H. Lange-Bertalot (Ed.), Diatoms of Europe: diatoms of the European inland waters and comparable habitats (2nd ed., p. 526). Ruggell: A.R.G. Gantner Verlag.
Lange-Bertalot, H., Hofmann, G., Werum, M., & Cantonati, M. (2017). Freshwater benthic diatoms of Central Europe: over 800 common species used in ecological assessment. In English edition with updated taxonomy and added species. Schmitten-Oberreifenberg: Koeltz Botanical Books.
Levkov, Z., Metzeltin, D., & Pavlov A. (2013). Luticola and Luticolopsis. In: H. Lange-Bertalot (Ed.), Diatoms of Europe. Diatoms of the European inland waters and comparable habitats. Volume 7 (p.698) Königstein: Koeltz Scientific Books.
Lowe, R. L., Kociolek, J. P., & Van de Vijver, B. (2013). Two new Orthoseira species (Bacillariophyceae) from lava tubes on Île Amsterdam and Big Island (Hawaii). Phytotaxa, 111(1), 39–52.
Lowe, R. L., Kociolek, P., Johansen, J. R., Van de Vijver, B., Lange-Bertalot, H., & Kopalová, K. (2014). Humidophila gen. nov., a new genus for a group of diatoms (Bacillariophyta) formerly within the genus Diadesmis: apecies from Hawai’i, including one new species. Diatom Research, 29(4), 351–360.
Mareš, J., Hrouzek, P., Kaňa, R., Ventura, S., Strunecký, O., & Komárek, J. (2013). The primitive thylakoid-less Cyanobacterium Gloeobacter is a common rock-dwelling organism. PLoS One, 8(6), e66323.
Miller, A. E., Steele, N., & Tobin, B. W. (2018). Vulnerability and fragility risk indices for non-renewable resources. Environmental Monitoring and Assessment, 190(7), 1–11.
Mulec, J. (2005). Algae in the karst caves of Slovenia. Doctoral thesis, University of Ljubljana, Ljubljana.
Mulec, J., Kosi, G., & Vrhovšek, D. (2008). Characterization of cave aerophytic algal communities and effects of irradiance levels on production of pigments. Journal of Cave and Karst Studies, 70(1), 3–12.
Mulec, J., & Kosi, G. (2009). Lampenflora algae and methods of growth control. Journal of Cave and Karst Studies, 71(2), 109–115.
Mulec, J. (2012). Lampenflora. In W. B. White & D. C. Culver (Eds.), Encyclopedia of caves (pp. 451–456). Amsterdam: Elsevier.
Mulec, J., Oarga-Mulec, A., Tomazin, R., & Matos, T. (2015). Characterization and fluorescence of yellow biofilms in karst caves, southwest Slovenia. International Journal of Speleology, 44(2), 107–114.
Pentecost, A., & Whitton, B. A. (2012). Subaerial Cyanobacteria. In B. A. Whitton (Ed.), Ecology of Cyanobacteria II: their diversity in space and time (pp. 291–316). London: Springer Science+Business Media B.V.
Popović, S., Subakov Simić, G., Stupar, M., Unković, N., Predojević, D., Jovanović, J., & Ljaljević Grbić, M. (2015). Cyanobacteria, algae and microfungi present in biofilm from Božana Cave (Serbia). International Journal of Speleology, 44(2), 141–149.
Popović, S., Subakov Simić, G., Stupar, M., Unković, N., Krunić, O., Savić, N., & Ljaljević Grbić, M. (2017). Cave biofilms: characterization of phototrophic cyanobacteria and algae and chemotrophic fungi from three caves in Serbia. Journal of Cave and Karst Studies, 79(1), 10–23.
Popović, S., Nikolić, N., Jovanović, J., Predojević, D., Trbojević, I., Manić, L., & Subakov Simić, G. (2019). Cyanobacterial and algal abundance and biomass in cave biofilms and relation to environmental and biofilm parameters. International Journal of Speleology, 48(1), 49–61.
Popović, S., Krizmanić, J., Vidaković, D., Jakovljević, O., Trbojević, I., Predojević, D., Vidović, M., & Subakov Simić, G. (2020). Seasonal dynamics of cyanobacteria and algae in biofilm from the entrance of two caves. Geomicrobiology Journal, 37(4), 315–326.
Rastogi, R. P., Sonani, R. R., & Madamwar, D. (2015). Cyanobacterial sunscreen scytonemin: role in photoprotection and biomedical research. Applied Biochemistry and Biotechnology, 176(6), 1551–1563.
Rindi, F. (2007). Diversity, distribution and ecology of green algae and cyanobacteria in urban habitats. In J. Seckbach (Ed.), Algae and cyanobacteria in extreme environments (pp. 621–638). Dordrecht: Springer.
Rochera, C., Fernández-Valiente, E., Van de Vijver, B., Rico, E., Toro, M., Vincent, W. F., Quesada, A., & Camacho, A. (2013). Community structure and photosynthetic activity of benthic biofilms from a waterfall in the maritime Antarctica. Polar Biology, 36, 1709–1722.
Roeselers, G. (2007). Microbial ecology of phototrophic biofilms. Doctoral thesis, Technische Universiteit Delft, Delft. ISBN 978-90-9022164-9.
Roldán, M., & Hernández-Mariné, M. (2009). Exploring the secrets of the three-dimensional architecture of phototrophic biofilms in caves. International Journal of Speleology, 38(1), 41–53.
Rossi, F., & De Philippis, R. (2015). Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life(Basel), 5(2), 1218–1238.
Rybak, M., Noga, T., & Zube, R. (2018). The Aerophytic diatom assemblages developed on mosses covering the bark of Populus alba L. Journal of Ecological Engineering, 19(6), 113–123.
Sanders, W. B. (2001). Lichens: the interface between mycology and plant morphology: whereas most other fungi live as an absorptive mycelium inside their food substrate, the lichen fungi construct a plant-like body within which photosynthetic algal symbionts are cultivated. BioScience, 51(12), 1025–1035.
Stal, L. J. (2012). Cyanobacterial mats and stromatolites. In B. A. Whitton (Ed.), Ecology of cyanobacteria II: their diversity in space and time (pp. 65–125). Dordrecht: Springer Science+Business Media B.V.
Sukenik, A., Hadas, O., Kaplan, A., & Quesada, A. (2012). Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes - physiological, regional, and global driving forces. Frontiers in Microbiology, 3, 86.
Taylor, J. C., De Le Rey, P. A., & Van Rensburg, L. (2005). Recommendations for the collection, preparation and enumeration of diatoms from riverine habitats for water quality monitoring in South Africa. African Journal of Aquatic Science, 30(1), 65–75.
Ter Braak, C. J. F., & Šmilauer, P. (2012). Canoco reference manual and user’s guide: software for ordination, version 5.0. Ithaca: Microcomputer Power.
Urzì, C., & De Leo, F. (2001). Sampling with adhesive tape strips: an easy and rapid method to monitor microbial colonization on monument surfaces. Journal of Microbiological Methods, 44(1), 1–11.
Wakefield, R. D., & Jones, M. S. (1998). An introduction to stone colonizing micro-organisms and biodeterioration of building stone. Quarterly Journal of Engineering Geology and Hydrogeology, 31(4), 301–313.
Werum, M., & Lange-Bertalot, H. (2004). Diatoms in springs, from Central Europe and elsewhere under the influence of hydrogeology and anthropogenic impacts. In Iconografia Diatomologica 13. Rugell: A.R.G. Gantner Verlag KG.
Wimpenny, J., Manz, W., & Szewzyk, U. (2000). Heterogeneity in biofilms. FEMS Microbiology Reviews, 24(5), 661–671.
Acknowledgments
The authors would like to express their gratitude to family, friends and cave managers for the support in biofilm sampling, Dr. Miljana Jakovljević for the help with figures, editor and the anonymous reviewers for valuable comments and suggestions which implementation improved the quality of this manuscript.
Funding
This research was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 451-03-68/2020-14/200026 and Grant No. 451-03-68/2020-14/200178).
Author information
Authors and Affiliations
Contributions
All authors have contributed to the project and all required criteria for authorship have been met.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Popović, S., Krizmanić, J., Vidaković, D. et al. Biofilms in caves: easy method for the assessment of dominant phototrophic groups/taxa in situ. Environ Monit Assess 192, 720 (2020). https://doi.org/10.1007/s10661-020-08686-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-08686-4