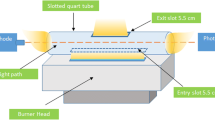
Abstract
Tellurium has been widely used in industrial processes and daily life products, and can cause serious health problems upon exposure. Therefore, determination of tellurium in real-life samples is very crucial. In this study, an accurate, environmentally friendly, and inexpensive analytical method was developed to determine trace levels of tellurium in water samples. To lower the detection limits, system parameters including flame type, acetylene flow rate, slotted quartz tube (T-SQT) height, and trapping period were optimized. Under the optimum conditions, the limit of detection (LOD) and quantification (LOQ) were calculated as 14.1 ng/mL and 47.1 ng/mL, respectively. For recovery studies, the optimized T-SQT-AT-FAAS method was applied to tap water samples to determine trace levels of tellurium and recovery results were found between 91.1 and 111.3%. Relative standard deviation value (%RSD) of the developed method was found to be less than 5.0% even for the lowest concentration in calibration plot, specifying good accuracy and high applicability of the method for water samples.

.



Similar content being viewed by others
References
Ba, L. A., Döring, M., Jamier, V., & Jacob, C. (2010). Tellurium: an element with great biological potency and potential. Organic & Biomolecular Chemistry, 8(19), 4203–4216.
Bingham, E., Cohrssen, B., & Powell, C. H. (2001). Patty’s toxicology. Volume 1: toxicology issues, inorganic particulates, dusts, products of biological origin and pathogens (Vol. Ed. 5): John Wiley and Sons.
Büyükpınar, Ç. (2017). Alevli Atomik Absorpsiyon Spektrofotometre Cihaz Duyarlılığının Yarıklı Kuartz Tüp (YKT) ile Artırılması ve Ykt İç Yüzeyinin Adsorpsiyon Özelliklerinin İncelenmesi. Istanbul: Yıldız Technical University.
de Quadros, D. P. C., & Borges, D. L. G. (2014). Direct analysis of alcoholic beverages for the determination of cobalt, nickel and tellurium by inductively coupled plasma mass spectrometry following photochemical vapor generation. Microchemical Journal, 116, 244–248. https://doi.org/10.1016/j.microc.2014.04.015.
Dedina, J. (1995). Hydride generation, atomic absorption spectrometry. Chemical Analysis, 130.
Ghasemi, E., Najafi, N. M., Seidi, S., Raofie, F., & Ghassempour, A. (2009). Speciation and determination of trace inorganic tellurium in environmental samples by electrodeposition-electrothermal atomic absorption spectroscopy. Journal of Analytical Atomic Spectrometry, 24(10), 1446–1451. https://doi.org/10.1039/B903162F. https://doi.org/10.1039/B903162F.
Guo, W., Shu, D., Chen, H., Li, A., Wang, H., Xiao, G., et al. (2009). Study on the structure and property of lead tellurium alloy as the positive grid of lead-acid batteries. Journal of Alloys and Compounds, 475(1–2), 102–109.
Ha, J., Sun, H.-W., Sun, J.-M., Zhang, D.-Q., & Yang, L.-L. (2001). Determination of tellurium in urine by hydride generation atomic absorption spectrometry with derivative signal processing. Analytica Chimica Acta, 448(1), 145–149. https://doi.org/10.1016/S0003-2670(01)01283-1.
Harada, T., & Takahashi, Y. (2008). Origin of the difference in the distribution behavior of tellurium and selenium in a soil–water system. Geochimica et Cosmochimica Acta, 72(5), 1281–1294.
Inglis-Arkell, E. (2018). Getting a tiny bit of this element on your skin will make you reek of garlic for weeks Accessed 2019.
Kaplan, M., Cerutti, S., Moyano, S., Olsina, R., Martinez, L., & Gásquez, J. (2004). On-line preconcentration system by coprecipitation with lanthanum hydroxide using packed-bed filter for the determination of tellurium in water by ICP-OES with USN. Instrumentation Science & Technology, 32(4), 423–431.
Kılınç, E., Bakırdere, S., Aydın, F., & Ataman, O. Y. (2012). Sensitive determination of bismuth by flame atomic absorption spectrometry using atom trapping in a slotted quartz tube and revolatilization with organic solvent pulse. Spectrochimica Acta Part B: Atomic Spectroscopy, 73, 84–88. https://doi.org/10.1016/j.sab.2012.06.004.
Leiber, M. A. (2005). Use of tellurium in carbon-supported, noble metal-containing catalysts for liquid phase oxidation reactions. Google Patents.
Ma, Y., Hao, Q., Poudel, B., Lan, Y., Yu, B., Wang, D., Chen, G., & Ren, Z. (2008). Enhanced thermoelectric figure-of-merit in p-type nanostructured bismuth antimony tellurium alloys made from elemental chunks. Nano Letters, 8(8), 2580–2584.
Merian, E., Anke, M., Ihnat, M., & Stoeppler, M. (2004). Elements and their compounds in the environment: occurrence, analysis and biological relevance (Vol. Ed. 2): Wiley-VCH Verlag GmbH & Co. KGaA.
Şahin, İ., Büyükpınar, Ç., San, N., & Bakırdere, S. (2018). Development of a sensitive analytical method for the determination of cadmium using hydrogen assisted T-shape slotted quartz tube-atom trap-flame atomic absorption spectrophotometry. Spectrochimica Acta Part B: Atomic Spectroscopy, 147, 9–12. https://doi.org/10.1016/j.sab.2018.05.017.
Shie, M. D., & Deeds, F. E. (1920). The importance of tellurium as a health hazard in industry. A preliminary report. Public Health Reports (1896-1970), 939–954.
Skoog, D. A., Holler, F. J., & Crouch, S. R. (2017). Principles of instrumental analysis: Cengage learning.
Taylor, A. (1996). Biochemistry of tellurium. Biological Trace Element Research, 55(3), 231–239.
Ürgüt, O. S. (2013). Antimon (III) halojenürlerin (SbX3 X: Bbr, I) tiyouram türevleri ile oluşturacakları yeni bileşiklerin sentezi, yapısal karakterizasyonu ve anti-tümör özelliklerinin incelenmesi. Namık Kemal Üniversitesi
Yaman, M., & Akdeniz, I. (2004). Sensitivity enhancement in flame atomic absorption spectrometry for determination of copper in human thyroid tissues. Analytical Sciences, 20(9), 1363–1366. https://doi.org/10.2116/analsci.20.1363.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Şahin, İ., Durak, B.Y., Sağsöz, O. et al. Optimization of T-shape slotted quartz tube with exit holes-atom trap-flame atomic absorption spectrophotometry system for the accurate and sensitive determination of tellurium in tap water. Environ Monit Assess 192, 61 (2020). https://doi.org/10.1007/s10661-019-8043-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-8043-6