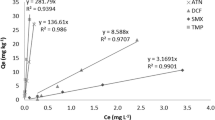
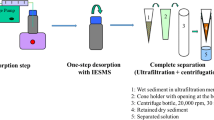
Abstract
Competitive sorption and desorption of Cd2+, Pb2+, and Hg2+ onto riverbank and sediment samples of an area impacted by pyritic residue in a Southern Brazilian catchment were evaluated. Although these ions are considered poorly mobile, a new approach has been proposed to assess their behavior and associated risk. In this sense, factorial design and three-dimensional surface methodology are proposed to describe the competitive sorption behavior of the metal ion in the environmental matrix, as well as an innovative mobilization factor (MF) to describe the desorption rate from the integration of the normalized difference of sorption-desorption fluorescence peaks. Sorption was carried out with a central composite factorial design (23) to estimate simultaneous effects of independent variables. Three-dimensional surface analysis indicated increasing Cd2+ equilibrium concentration (Ceq) with Hg2+ and Pb2+ initial concentration (Ci), showing synergistic effect and low Cd2+ affinity to the solid phase. Statistical analysis presented \(C_{i_{\text {Hg}}}\) as a significant variable for cadmium and lead dynamics, although \(C_{i_{\text {Pb}}}\) was also significant for Hg2+ releasing to the liquid phase. After integrating the sorption and desorption fluorescence peaks, the MF for Cd2+, Pb2+, and Hg2+ was around 0.2, 0.5, and 0.1 in riverbank sediment, and 0.3, 0.9, and 0.1 in sediment, respectively. Hence, consistent ion mobilization along the river was observed, with Pb2+ mobilizing 9 and 6 times more than Hg2+ and Cd2+, respectively. The transport of ions such as Pb2+ and Hg2+, usually considered immobile, has indeed occurred, causing contamination through the watershed and increasing environmental risk.

A new approach to determine toxic metal mobilization factor in a river catchment.





Similar content being viewed by others
References
Ahmadi, M., Vahabzadeh, F., Bonakdarpour, B., Mofarrah, E., Mehranian, M. (2005). Application of the central composite design and response surface methodology to the advanced treatment of olive oil processing wastewater using Fenton’s peroxidation. Journal of Hazardous Materials, 123(1), 187–195.
Ali, H., Khan, E., Sajad, M. (2013). Phytoremediation of heavy metals - concepts and applications. Chemosphere, 91, 869–881.
ATSDR. (2015). Priority List of Hazardous Substances. Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services. Public Health Service, Division of Toxicology and Human Health Sciences (proposed). Environmental Toxicology Branch (proposed). 1600 Clifton road NE. Mailstop f-62. Atlanta, Georgia 30333.
Baird, C. (2012). Environmental chemistry, 5th edn. New York: W.H. Freeman and Company.
Bezerra, M., Santelli, E.R., Oliveira, E., villar, L., escaleira, L. (2008). Response surface methodology (RSM,) as a tool for optimization in analytical chemistry. Talanta, 76(5), 965–977.
Bingol, D., Saraydin, D., Ozbay, D.S. (2015). Full factorial design approach to Hg(II) adsorption onto hydrogels. Arabian Journal for Science and Engineering, 40(1), 109–116.
Bolan, N., Kunhikrishnanc, A., Thangarajana, R., Kumpiened, J., Parke, J., Makinof, T., Kirkhamg, M., Scheckelh, K. (2014). Remediation of heavy metal(loid)s contaminated soils - to mobilize or to immobilize?. Journal of Hazardous Materials, 266, 141–166.
Box, G., Hunter, J., Hunter, W. (2005). Statistics for experimenters: design, innovation, and discovery, 2nd edn. New Jersey: Wiley-Interscience.
Bui, A., Nguyen, H., Nguyen, M., Tran, T., Vu, T., Nguyen, C., Reynolds, H. (2016). Accumulation and potential health risks of cadmium, lead and arsenic in vegetables grown near mining sites in Northern Vietnam. Environmental monitoring and assessment, 188(9), 525.
Butler, B. (2009). Effect of pH, ionic strength, dissolved organic carbon, time, and particle size on metals release from mine drainage impacted streambed sediments. Water Research, 43(5), 1392–1402.
Cesar, R., Egler, S., Polivanov, H., Castilhos, Z., Rodrigues, A. (2011). Mercury, copper and zinc contamination in soils and fluvial sediments from an abandoned gold mining area in southern Minas Gerais State, Brazil. Environmental Earth Sciences, 64(1), 211–222.
Cesareo, R. (2010). X-ray fluorescence spectrometry. Hoboken: Wiley Online Library.
Cho, I., & Zoh, K. (2007). Photocatalytic degradation of azo dye (Reactive Red 120) in TiO 2/UV system: optimization and modeling using a response surface methodology (RSM) based on the central composite design. Dyes and Pigments, 75(3), 533–543.
Chotpantarat, S., Ong, S., Sutthirat, C., Osathaphan, K. (2011). Competitive sorption and transport of Pb2+, Ni2+, Mn2+, and Zn2+ in lateritic soil columns. Journal of Hazardous Materials, 190(1), 391–396.
Consani, S., Carbone, C., Dinelli, E., Balić-žunić, T., Cutroneo, L., Capello, M., Salviulo, G., Lucchetti, G. (2017). Metal transport and remobilisation in a basin affected by acid mine drainage: the role of ochreous amorphous precipitates. Environmental science and pollution Research, 1–13.
Constantino, L., Quirino, J., Monteiro, A., Abrao, T., Parreira, P., Urbano, A., Santos, M. (2017). Sorption-desorption of selenite and selenate on Mg-Al layered double hydroxide in competition with nitrate, sulfate and phosphate. Chemosphere, 181, 627–634.
de Oliveira, L., Cabral, M., Vieira, C., Antoniazzi, M., Risso, W., dos Reis Martinez, C. (2016). Metals bioaccumulation and biomarkers responses in the neotropical freshwater clam anodontites trapesialis: implications for monitoring coal mining areas. The Science of the Total Environment, 571, 983–991.
de Oliveira, L., Cabral, M., Risso, W., dos Reis Martinez, C. (2018a). Single and combined effects of Zn, Mn and Fe on the neotropical freshwater bivalve anodontites trapesialis: bioaccumulation and biochemical biomarkers. Ecotoxic. Environ. Safety, 161, 735–745.
de Oliveira, L., Santos, C., Risso, W., dos Reis Martinez, C. (2018b). Triple-mixture of Zn, Mn, and Fe increases bioaccumulation and causes oxidative stress in freshwater neotropical fish. Environmental Toxicology and Chemistry, 37(6), 1749–1756.
Dragovic, S., Mihailovic, N., Gajic, B. (2008). Heavy metals in soils: distribution, relationship with soil characteristics and radionuclides and multivariate assessment of contamination sources. Chemosphere, 72(3), 491–495.
Echeverria, J., Morera, M., Mazkiaran, C., Garrido, J. (1998). Competitive sorption of heavy metal by soils. isotherms and fractional factorial experiments. Environmental Pollution, 101(2), 275–284.
Eckert, D., & Sims, J. (1995). Recommended soil pH and lime requirement tests. Recommended soil testing procedures for the northeastern United States. Northeast Regional Bulletin, 493, 11–16.
Embrapa. (1997). Handbook for methods of soil chemical analyses, 2nd edn. Rio de Janeiro: The Brazilian Company for Agricultural Research, National Center of Soil Research.
Essington, M. (2015). Soil and water chemistry, 2nd edn. New York: CRC press.
Fiol, N., & Villaescusa, I. (2009). Determination of sorbent point zero charge: usefulness in sorption studies. environmental Chemistry Letters, 7(1), 79–84.
Gagnon, C., Arnac, M., Brindle, J.R. (1992). Sorption interactions between trace metals (Cd and Ni) and phenolic substances on suspended clay minerals. Water Research, 26(8), 1067–1072.
Galunin, E., Ferreti, J., Zapelini, I., Vieira, I., Tarley, C., Abrao, T., Santos, M. (2014). Cadmium mobility in sediments and soils from a coal mining area on Tibagi River watershed: Environmental risk assessment. Journal of Hazardous Materials, 265, 280–287.
Gernet, M., Melo, V., Dieckow, J., Lima, V., Silva, W. (2014). Genesis and occupancy of a shell midden on Paraná State coast. Brazilian Quaternary International, 352, 135–146.
Hu, N., Liu, J., Huang, P., Yan, S., Shi, X., Ma, D. (2017). Sources, geochemical speciation, and risk assessment of metals in coastal sediments: a case study in the Bohai Sea, China. Environmental Earth Sciences, 76(8), 309.
Kabata-Pendias, A. (2010). Trace elements in soils and plants, 4th edn. New York: CRC Press.
Landis, W., & Yu, M. (2003). Introduction to environmental toxicology: impacts of chemicals upon ecological systems, 3rd edn. USA: Lewis Publisher.
Li, Z., Ma, Z., van der Kuijp, T., Yuan, Z., Huang, L. (2014). A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Science of the Total Environment, 468, 843–853.
Li, N., Tian, Y., Zhang, J., Zuo, W., Zhan, W., Zhang, J. (2017). Heavy metal contamination status and source apportionment in sediments of Songhua River Harbin region, Northeast China. Environmental Science and Pollution Research, 24(4), 3214–3225.
Liao, J., Wen, Z., Ru, X., Chen, J., Wu, H., Wei, C. (2016). Distribution and migration of heavy metals in soil and crops affected by acid mine drainage: public health implications in Guangdong Province, China. Ecotoxicity and Environmental Safety, 124, 460–469.
Liu, W., Wang, T., Borthwick, A., Wang, Y., Yin, X., Li, X., Ni, J. (2013). Adsorption of Pb2+, Cd2+, Cu2+ and Cr3+ onto titanate nanotubes: competition and effect of inorganic ions. Science of Total Environment, 456, 171–180.
Lu, S., Wang, Y., Teng, Y., Yu, X. (2015). Heavy metal pollution and ecological risk assessment of the paddy soils near a zinc-lead mining area in Hunan. Environmental monitoring and assessment, 187 (10), 627.
May, P., & Bulman, R. (1983). The present status of chelating agents in medicine. Progress in Medicinal Chemistry, 20, 225–336.
McComb, J., Rogers, C., Han, F., Tchounwou, P. (2014). Rapid screening of heavy metals and trace elements in environmental samples using portable X-ray fluorescence spectrometer: a comparative study. Water, Air, & Soil Pollution, 225(12), 2169.
Melquiades, F., & Appoloni, C. (2004). Application of XRF and field portable XRF for environmental analysis. Journal of radioanalytical and nuclear chemistry, 262(2), 533–541.
Melquiades, F., Parreira, P., Appoloni, C., Silva, W., Lopes, F. (2011). Quantification of metals in river water using a portable EDXRF system. Applied Radiation and Isotopes, 69(2), 327–333.
Ming, H., Naidu, R., Sarkar, B., Lamb, D., Liu, Y., Megharaj, M., Sparks, D. (2016). Competitive sorption of cadmium and zinc in contrasting soils. Geoderma, 268, 60–68.
Mouni, L., Belkhiri, L., Bouzaza, A., Bollinger, J. (2017). Interactions between Cd, Cu, Pb, and Zn and four different mine soils. Arabian Journal of Geosciences, 10(4), 77.
Nascimento, V., Almendros, G., Fernandes, F. (1992). Soil humus characteristics in virgin and cleared areas of the Paraná River basin in Brazil. Geoderma, 54(1), 137–150.
Pansu, M., & Gautheyrou, J. (2007). Handbook of soil analysis: mineralogical, organic and inorganic methods. Springer Science & Business Media.
Pareja-Carrera, J., Mateo, R., Rodríguez-estival, J. (2014). Lead (Pb) in sheep exposed to mining pollution: implications for animal and human health. Ecotoxicology and environmental safety, 108, 210–216.
Park, J., Ok, Y., Kim, S., Cho, J., Heo, J., Delaune, R., Seo, D. (2016). Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere, 142, 77–83. Biochars multifunctional role as a novel technology in the agricultural, environmental, and industrial sectors.
Pérez-Serradilla, J., & de Castro, M. (2007). Integrated sorption-energy-dispersive X-ray fluorescence detection for automatic determination of lead and cadmium in low-concentration solutions. Analytical and bioanalytical chemistry, 389(5), 1541–1547.
Santos, M.J. (2018). Dataset: metal mobilization factor modeling. Mendeley Data Set. Available at http://data.mendeley.com/datasets/kmpv8s7nbk/1.
Santos, M., Tarley, C., Cunha, I., Zapelini, I., Galunin, E., Bleinroth, D., Vieira, I., Abrao, T. (2015). Leachability of major and minor elements from soils and sediments of an abandoned coal mining area in southern Brazil. Environmental Monitoring and Assessment, 187(3), 83.
Sarkar, D. (2005). Physical and chemical methods in soil analysis. New Age International.
Selim, H. (2012). Competitive sorption and transport of heavy metals in soils and geological media. CRC Press.
Seo, D., Yu, K., DeLaune, R. (2008). Comparison of monometal and multimetal adsorption in Mississippi River alluvial wetland sediment: batch and column experiments. Chemosphere, 73(11), 1757–1764.
Serrano, S., O Day, P., Vlassopoulos, D., García-gonzález, M., Garrido, F. (2009). A surface complexation and ion exchange model of Pb and Cd competitive sorption on natural soils. Geochimica et Cosmochimica Acta, 73(3), 543–558.
Silbergeld, E.K., Waalkes, M., Rice, J.M. (2000). Lead as a carcinogen: experimental evidence and mechanisms of action. American Journal Industrial Medicine, 38(3), 316–323.
Silva, L., de Vallejuelo, S., Martinez-Arkarazo, I., Castro, K., Oliveira, M., Sampaio, C., de Brum, I., De leao, F., Taffarel, S., Madariaga, J. (2013). Study of environmental pollution and mineralogical characterization of sediment rivers from Brazilian coal mining acid drainage. Science of the total environment, 447, 169–178.
Simate, G., & Ndlovu, S. (2014). Acid mine drainage: challenges and opportunities. Journal of Environmental Chemical Engineering, 2(3), 1785–1803.
Tagami, K., Twining, J.R., Wasserman, M. (2012). Tropical radioecology, chapter Terrestrial radioecology in tropical systems, (pp. 155–230). Amsterdam: Elsevier.
Tan, K. (2010). Principles of soil chemistry. Boca Raton: CRC press.
Tansel, B., & Dizge, N. (2011). Competitive effects and interactions during sorption of SMP fractions on activated carbon: response surface approach for visualization of sorption profiles. Journal of Environmental Management, 92, 596– 602.
USDA. (2017a). Soil Survey Manual. Soil Science Division Staff, 4th edn. Washington: United States Department of Agriculture. Handbook No. 18.
USDA. (2017b). Soil Texture Calculator. Natural Resources Conservation Service Soils. United States Department of Agriculture.
Verma, H. (2007). Atomic and nuclear analytical methods: XRF, mössbauer, XPS, NAA and B63Ion-Beamspectroscopic Techniques. Berlin: Springer.
Vidal, M., Santos, M., Abrao, T., Rodríguez, J., Rigol, A. (2009). Modeling competitive metal sorption in a mineral soil. Geoderma, 149(3), 189–198.
Waalkes, M. (2003). Review cadmium carcinogenesis. Mutation Research, 533, 107–120.
Witek-Krowiak, A., Chojnacka, K., Podstawczyk, D., Dawiec, A., Pokomeda, K. (2014). Application of response surface methodology and artificial neural network methods in modelling and optimization of biosorption process. Bioresource Technology, 160, 150–160.
Wuana, R.A., & Okieimen, F. (2011). Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Isrn Ecology.
Yu, M., Tsunoda, H., Tsunoda, M. (2016). Environmental toxicology biological and health effects of pollutants, 3rd edn. Routledge: Taylor & Francis Group.
Zhao, H., Xia, B., Fan, C., Zhao, P., Shen, S. (2012). Human health risk from soil heavy metal contamination under different land uses near Dabaoshan Mine, Southern China. Science of the Total Environment, 417, 45–54.
Zuluaga Rodríguez, J., Gallego Rios, S., Ramírez Botero, C. (2015). Content of Hg, Cd, Pb and As in fish species: a review. Vitae, 22(2), 148–149.
Acknowledgments
The authors would like to thank prof. A. Urbano from Physics Department, Universidade Estadual de Londrina, for the insightful discussions on the X-ray diffraction.
Funding
This study received financial support and fellowships from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), under Grants 309927/2015-3 and 304066/2015-0 (Researcher grant), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), and Fundação Araucária (FAP-FA, Brazil) under Grant 507/2014 (Researcher grant).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
1. Competitive sorption by applying factorial design and EDXRF was evaluated;
2. 3-D surface model indicates Cd2+ and Pb2+ synergistic effect on Hg2+ mobilization;
3. EDXRF analysis indicated high Pb2+ mobilization through the basin;
4. Innovative mobilization factor determined from the fluorescence peaks integration;
5. Even with specific interactions, Hg2+ ions were mobilized.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Frachini, E., Constantino, L.V., Abrao, T. et al. A new approach to evaluate toxic metal transport in a catchment. Environ Monit Assess 192, 234 (2020). https://doi.org/10.1007/s10661-019-7950-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7950-x