Abstract
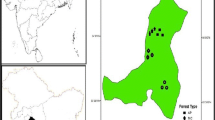
Environmental factors along with soil physico-chemical properties play a significant role on the diurnal trend of soil CO2 efflux. Soil CO2 efflux in Indian tropical forests is poorly studied. We studied the soil CO2 efflux in a representative tropical deciduous forest at Katerniaghat Wildlife Sanctuary (KWLS), Uttar Pradesh. The three forest communities namely dry mixed (DMF), Sal mixed (SMF), and Teak plantation (TPF) were selected for measuring soil CO2 efflux in the summer season during April to May 2017 using automated LI-COR 8100 soil CO2 flux system. Soil physico-chemical parameters were also studied in the three abovementioned forest communities. We also measured the different microclimatic variables at forest understorey in all three communities during the summer season. Total day time soil CO2 efflux of 826.70, 1089.24, and 828.94 (μmolCO2 m−2d−1) was observed in TPF, SMF, and DMF respectively. Soil CO2 efflux observed significant differences (P < 0.01) among the three forest communities studied for the summer season in tropical deciduous forest of Terai Himalaya. Average soil CO2 efflux rate (μmol CO2 m−2 s−1) of 4.06 ± 0.36, 5.03 ± 0.45, and 4.37 ± 0.79 was observed in TPF, SMF, and DMF, respectively, which is positively correlated with total organic carbon (TOC) and water holding capacity (WHC) among soil physico-chemical variables. Among microclimatic variables, soil temperature (ST, °C) and air temperature (AT, °C) observed strong positive correlation with day time soil CO2 efflux in all three communities. Significant increase in soil CO2 flux was observed with increasing air and soil temperature (AT and ST) in DMF and SMF. Maximum TOC of 19.23 g Kg−1 was observed in SMF among all communities in the summer season. The result showed that soil CO2 efflux is closely associated with TOC, WHC, AT, and ST for Indian deciduous forest ecosystems.





Similar content being viewed by others
References
Acosta, M., Darenova, E., Krupková, L., & Pavelka, M. (2018). Seasonal and inter-annual variability of soil CO2 efflux in a Norway spruce forest over an eight-year study. Agricultural and Forest Meteorology, 256, 93–103.
Ahirwal, J., Maiti S.K. & Singh A. K. (2017). Changes in ecosystem carbon pool and soil CO2 flux following post-mine reclamation in dry tropical environment. India Science of the Total Environment, 6-10. https://doi.org/10.1016/j.scitotenv.2017.01.043.
Almagro, M., López, J., Querejeta, J., & Martínez-Mena, M. (2009). Temperature dependence of soil CO2 efflux is strongly modulated by seasonal patterns of moisture availability in a Mediterranean ecosystem. Soil Biology and Biochemistry, 41(3), 594–605.
Anderson, J., & Ingram, J. (1993). A handbook of methods (p. 221). Wallingford: CAB International.
Andrew, N. P., Barrett, J. E., Diana, H. W., & Ross, A. V. (2004). Soil carbon dioxide flux in Antarctic dry valley ecosystems. Ecosystems, 7, 286–295.
Arora, P., & Chaudhry, S. (2017). Dependency of rate of soil respiration on soil parameters and climatic factors in different tree plantations at Kurukshetra, India. Tropical Ecology, 58(3), 573–581 ISSN 0564-3295.
Arshad, M. A., Lowery, B., & Grossman, B. (1996). Physical tests for monitoring soil quality. Methods for assessing soil quality (pp. 123-141). Soil Science Society of America Special Publication 49, USA.
Bajpai, O., Kumar, A., Mishra, A. K., Sahu, N., Behera, S. K., & Chaudhary, L. B. (2012). Phenological study of two dominant tree species in tropical moist deciduous forest from the Northern India. International Journal of Botany, 8(2), 66–72.
Behera, S. K., Mishra, A. K., Sahu, N., Kumar, A., Singh, N., Kumar, A., & Tuli, R. (2012). The study of microclimate in response to different plant community association in tropical moist deciduous forest from northern India. Biodiversity and Conservation, 21(5), 1159–1176.
Berisso, F. E., Schjønning, P., Keller, T., Lamandé, M., Simojoki, A., Iversen, B. V., & Forkman, J. (2013). Gas transport and subsoil pore characteristics: anisotropy and long-term effects of compaction. Geoderma, 195, 184–191.
Blanco-Canqui, H. (2010). Energy crops and their implications on soil and environment. Agronomy Journal, 102(2), 403–419.
Cavaleri, M. A., Reed, S. C., Smith, W. K., & Wood, T. E. (2015). Urgent need for warming experiments in tropical forests. Global Change Biology, 21(6), 2111–2121.
Chambers, J. Q., Tribuzy, E. S., Toledo, L. C., Crispim, B. F., Higuchi, N., Santos, J. D., & Trumbore, S. E. (2004). Respiration from a tropical forest ecosystem: partitioning of sources and low carbon use efficiency. Ecological Applications, 14(sp4), 72–88.
Chauhan, D., Dhanai, C., Singh, B., Chauhan, S., Todaria, N., & Khalid, M. (2008). Regeneration and tree diversity in natural and planted forests in a Terai-Bhabhar forest in Katarniaghat wildlife sanctuary, India. Tropical Ecology, 49(1), 53–67.
Chen, J., Saunders, S. C., Crow, T. R., Naiman, R. J., Brosofske, K. D., Mroz, G. D., & Franklin, J. F. (1999). Microclimate in forest ecosystem and landscape ecology: variations in local climate can be used to monitor and compare the effects of different management regimes. BioScience, 49(4), 288–297.
Chen, J., Falk, M., Euskirchen, E., Paw, U., U, K. T., Suchanek, T. H., Ustin, S. L., & Bi, R. (2002). Biophysical controls of carbon flows in three successional Douglas-fir stands based on eddy-covariance measurements. Tree Physiology, 22(2-3), 169–177.
Cleveland, C. C., & Townsend, A. R. (2006). Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proceedings of the National Academy of Sciences, 103(27), 10316–10321.
Coutinho, L. M., & Lamberti, A. (1971). Respiração Edáfica e Produtividade Primária numa Comunidade Amazônica de Mata de Terra-firme. Ciência e Cultura, 23, 411–419.
Das, S., Ganguly, D., Ray, R., Jana, T. K., & De, T. K. (2017). Microbial activity determining soil CO2 emission in the Sundarban mangrove forest, India. Tropical Ecology, 58(3), 525–537.
Davidson, E. A., Verchot, L. V., Cattanio, J. H., Ackerman, I. L., & Carvalho, J. (2000). Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry, 48(1), 53–69.
Davidson, E. A., Savage, K., Bolstad, P., Clark, D. A., Curtis, P. S., Ellsworth, D. S., Hanson, P. J., Law, B. E., Luo, Y., Pregitzer, K. S., Randolph, J. C., & Zak, D. (2002). Belowground carbon allocation in forests estimated from litter fall and IRGA-based soil respiration measurements. Agricultural and Forest Meteorology, 113, 39–51.
Devi, N. B., & Yadava, P. S. (2008). Emission of CO2 from soil in a subtropical mixed oak forest of Manipur, North-eastern India. International Journal of Ecology and Environmental Sciences, 34(3), 293–297.
Devi, M. D., & Yadava, P.S. (2015). Abiotic factor influences soil carbon dioxide flux in the sub-tropical forest, Manipur, NE India. International Journal ofScientific Research, 4(5), 3-5.
Doff Sotta, E., Meir, P., Malhi, Y., Donato Nobre, A., Hodnett, M., & Grace, J. (2004). Soil CO2 efflux in a tropical forest in the central Amazon. Global Change Biology, 10(5), 601–617.
Epron, D., Farque, L., Lucot, É., & Badot, P.-M. (1999). Soil CO2 efflux in a beech forest: dependence on soil temperature and soil water content. Annals of Forest Science, 56(3), 221–226.
Epron, D., Ngao, J., & Granier, A. (2004). Interannual variation of soil respiration in a beech forest ecosystem over a six-year study. Annals of Forest Science, 61(6), 499-505.
Evanylo, G. K., & McGuinn, R. (2000). Agricultural management practices and soil quality: measuring, assessing, and comparing laboratory and field test kit indicators of soil quality atributes. Virginia Cooperative Extension, Virginia Tech University, Publication 452-400.
Fan, S. M., Wofsy, S. C., Bakwin, P. S., Jacob, D. J., & Fitzjarrald, D. R. (1990). Atmosphere-biosphere exchange of CO2 and O3 in the central Amazon forest. Journal of Geophysical Research-Atmospheres, 95(D10), 16851–16864.
Goulden, M. L., Miller, S. D., Da Rocha, H. R., Menton, M. C., de Freitas, H. C., Silva Figueira, A. M., & de Sousa, C. A. D. (2004). Diel and seasonal patterns of tropical forest CO2 exchange. Ecological Applications, 14(sp4), 42–54.
IGBP Terrestrial Carbon Working Group (1998). The terrestrial carbon cycle: implications for the Kyoto Protocol.
Hammer, Ø., Harper, D. A., & Ryan, P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 9.
Hanson, P., Edwards, N., Garten, C. T., & Andrews, J. (2000). Separating root and soil microbial contributions to soil respiration: a review of methods and observations. Biogeochemistry, 48(1), 115–146.
Jenkinson, D. S., Adams, D., & Wild, A. (1991). Model estimates of CO2 emissions from soil in response to global warming. Nature, 351(6324), 304–306.
Jha, R. (2000). Management plan of Katarniaghat Wildlife Sanctuary (2000–2001 to 2009–2010). Uttar Pradesh: Wildlife Preservation Organisation. Forest Department.
Kätterer, T., Reichstein, M., Andrén, O., & Lomander, A. (1998). Temperature dependence of organic matter decomposition: a critical review using literature data analyzed with different models. Biology and Fertility of Soils, 27(3), 258–262.
Keen, B. A., & Raczkowski, H. (1921). The relation between the clay content and certain physical properties of a soil. The Journal of Agricultural Science, 11(4), 441–449.
Keith, H., Jacobsen, K., & Raison, R. (1997). Effects of soil phosphorus availability, temperature and moisture on soil respiration in Eucalyptus pauciflora forest. Plant and Soil, 190(1), 127–141.
Kepler, S., Volkoff, B., Cerri, C. C., Chone, T., Luizão, F., & Eduardo, B. P. (1990). Respiração do solo: comparação entre áreas com mata natural, mata recém queimada e pastagem, na Amazônia central. Geochimica Brasiliensis, 4(2), 111-118.
Koizumi, H., Kontturi, M., Mariko, S., Nakadai, T., Bekku, Y., & Mela, T. (1999). Soil respiration in three soil types in agricultural ecosystems in Finland. Acta Agriculturae Scandinavica, Section B-Plant Soil Science, 49(2), 65–74.
Landsberg, J. J., & Gower, S. T. (1997). Applications of physiological ecology to forest management. Elsevier.
Lee, H., Schuur, E. A., & Vogel, J. G. (2006). Soil carbon dioxide fluxes in subarctic tundra where permafrost is thawing. American Geophysical Union Fall Meeting 2006, Abstract id GC51A-0440.
Lewis, S. L., Lopez-Gonzalez, G., Sonké, B., Affum-Baffoe, K., Baker, T. R., Ojo, L. O., & Comiskey, J. A. (2009). Increasing carbon storage in intact African tropical forests. Nature, 457(7232), 1003–1006.
Liang, N., Teramoto, M., Takagi, M., & Zeng, J. (2017). High-resolution data on the impact of warming on soil CO2 efflux from an Asian monsoon forest. Scientific Data, 4, 170026.
Logsdon, S. D., & Karlen, D. L. (2004). Bulk density as a soil quality indicator during conversion to no-tillage. Soil and Tillage Research, 78(2), 143–149.
Luan, J., Liu, S., Zhu, X., Wang, J., & Liu, K. (2012). Roles of biotic and abiotic variables in determining spatial variation of soil respiration in secondary oak and planted pine forests. Soil Biology & Biochemistry, 44, 143–150.
Luo, Y., & Zhou, X. (2006). Soil respiration and the environment. Amsterdam: Elsevier/Academic Press 316 pp.
Malhi, Y., Silman, M., Salinas, N., Bush, M., Meir, P., & Saatchi, S. (2010). Introduction: elevation gradients in the tropics: laboratories for ecosystem ecology and global change research. Global Change Biology, 16(12), 3171–3175.
Mande, H. K., Abdullah, A. M., Aris, A. Z., & Ainuddin, A. N. (2015). Factors responsible for spatial and temporal variation of soil CO2 efflux in a 50 year recovering tropical forest, Peninsular Malaysia. Environmental Earth Sciences, 73(9), 5559–5569.
Martins, F. R., & Matthes, L. A. F. (1978). Respiração edáfica e nutrientes na Amazônia (Região de Manaus): floresta arenícola, campinarana e campina. Acta Amazonica, 8(2), 233–244.
Medina, E., Klinge, H., Jordan, C., & Herrera, R. (1980). Soil respiration in Amazonian rain forests in the Rio Negro Basin. Flora, 170(3), 240–250.
Meir, P. (1996). Soil respiration in a rainforest in Amazonia and in cerrado in central Brazil. Amazonian deforestation and climate. Wiley.
Mo, W. H., Lee, M. S., Uchida, M., Inatomi, M., Saigusa, N., Mariko, S., & Koizumi, H. (2005). Seasonal and annual variations in soil respiration in a cool-temperate deciduous broad-leaved forest in Japan. Agricultural and Forest Meteorology, 134, 81–94.
Ngao, J., Epron, D., Delpierre, N., Bréda, N., Granier, A., & Longdoz, B. (2012). Spatial variability of soil CO2 efflux linked to soil parameters and ecosystem characteristics in a temperate beech forest. Agricultural and Forest Meteorology, 154, 136–146.
Nsabimana, D., Klemedtson, L., Kaplin, B., & Wallin, G. (2009). Soil CO2 flux in six monospecific forest plantations in Southern Rwanda. Soil Biology and Biochemistry, 41(2), 396–402.
Parker, L. W., Santos, P. F., Phillips, J., & Whitford, W. G. (1984). Carbon and nitrogen dynamics during the decomposition of litter and roots of a Chihuahuan desert annual, Lepidium lasiocarpum. Ecological Monographs, 54(3), 339–360.
Pendall, E., Bridgham, S., Hanson, P. J., Hungate, B., Kicklighter, D. W., Johnson, D. W., & Olsrud, M. (2004). Below-ground process responses to elevated CO2 and temperature: a discussion of observations, measurement methods, and models. New Phytologist, 162(2), 311–322.
Raich, J. W., & Schlesinger, W. H. (1992). The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B, 44(2), 81–99.
Raich, J. W., & Tufekciogul, A. (2000). Vegetation and soil respiration: correlations and controls. Biogeochemistry, 48(1), 71–90.
Reed, S. C., Cleveland, C. C., & Townsend, A. R. (2008). Tree species control rates of free-living nitrogen fixation in a tropical rain forest. Ecology, 89(10), 2924–2934.
Rhoades, J. D. (1982). Cation Exchange Capacity 1. Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy, Soil Science Society of America (pp. 149-157).https://doi.org/10.2134/agronmonogr9.2.2ed.c8.
Robertson, G. P., Dale, V. H., Doering, O. C., Hamburg, S. P., Melillo, J. M., Wander, M. M., & Cruse, R. M. (2008). Sustainable biofuels redux. Science, 322(5898), 49–50.
Rodgers, W. A., & Panwar, H. S. (1988). Planning a wildlife protected area network in India. Food and Agriculture Organization of the United Nations.
Salimon, C., Davidson, E., Victoria, R., & Melo, A. (2004). CO2 flux from soil in pastures and forests in southwestern Amazonia. Global Change Biology, 10(5), 833–843.
Sayer, J., Campbell, B., Petheram, L., Aldrich, M., Perez, M. R., Endamana, D., & Doggart, N. (2007). Assessing environment and development outcomes in conservation landscapes. Biodiversity and Conservation, 16(9), 2677–2694.
Schlesinger, W. H. (1977). Carbon balance in terrestrial detritus. Annual Review of Ecology and Systematics, 8(1), 51–81.
Schwendenmann, L., & Macinnis-Ng, C. (2016). Soil CO2 efflux in an old-growth southern conifer forest (Agathis australis)–magnitude, components and controls. Soil, 2(3), 403–419.
Schwendenmann, L., Veldkamp, E., Brenes, T., O’Brien, J. J., & Mackensen, J. (2003). Spatial and temporal variation in soil CO2 efflux in an old-growth neotropical rain forest, La Selva, Costa Rica. Biogeochemistry, 64(1), 111–128.
Shi, P.-L., Zhang, X.-Z., Zhong, Z.-M., & Ouyang, H. (2006). Diurnal and seasonal variability of soil CO2 efflux in a cropland ecosystem on the Tibetan Plateau. Agricultural and Forest Meteorology, 137(3-4), 220–233.
Solomon, S. (2007). The physical science basis: Contribution of Working Group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Intergovernmental Panel on Climate Change (IPCC), Climate change 2007, 996, Cambridge University Press.
Souza, J. S. D. (2004). Dinamica espacial e temporal do fluxo de CO2 do solo em floresta de terra firme na amazonia central. Master Thesis, Universidad Federal do Amazonas, Manaus.
Sundarapandian, S. M., & Dar, J. A. (2013). Variation in soil CO2 efflux in Pinus wallichiana and Abies pindrow temperate forests of Western Himalayas, India. Journal of Forest Research, 3, 116. https://doi.org/10.4172/2168-9776.1000116.
Tanaka, N., Hamazaki, T., & Vacharangkura, T. (1998). Distribution, growth and site requirements of teak. Japan Agricultural Research Quarterly, 32, 65-77.
Thomas, G. W. (1996). Soil pH and soil acidity. Methods of Soil Analysis: Part 3-Chemical Methods (pp. 475–490). Madison, WI. Soil Science Society of America Book Series 5.
Tripathi, K., & Singh, B. (2009). Species diversity and vegetation structure across various strata in natural and plantation forests in Katerniaghat Wildlife Sanctuary, North India. Tropical Ecology, 50(1), 191.
Trumbore, S. E., Davidson, E. A., Barbosa de Camargo, P., Nepstad, D. C., & Martinelli, L. A. (1995). Belowground cycling of carbon in forests and pastures of Eastern Amazonia. Global Biogeochemical Cycles, 9(4), 515–528.
Valentini, C. M. A., Sanches, L., de Paula, S. R., Vourlitis, G. L., de Souza Nogueira, J., Pinto, O. B., & de Almeida Lobo, F. (2008). Soil respiration and aboveground litter dynamics of a tropical transitional forest in northwest Mato Grosso, Brazil. Journal of Geophysical Research, 113 G00B10. https://doi.org/10.1029/2007JG000619.
Valverde-Barrantes, O. J. (2007). Relationships among litterfall, fine-root growth, and soil respiration for five tropical tree species. Canadian Journal of Forest Research, 37(10), 1954–1965.
Wang, C., Yang, J., & Zhang, Q. (2006). Soil respiration in six temperate forests in China. Global Change Biology, 12(11), 2103–2114.
Wangdi, N., Mayer, M., Nirola, M. P., Zangmo, N., Orong, K., Ahmed, I. U., Darabant, A., Jandl, R., Gratzer, G., & Schindlbacher, A. (2017). Soil CO2 efflux from two mountain forests in the eastern Himalayas, Bhutan: components and controls. Biogeosciences, 14, 99–110.
Wen-De, Y., Wang-Ming, X., Xiao-Yong, C., Da-Lun, T., Yuan-Ying, P., Wei, Z., & Jie, X. (2014). Soil CO2 flux in different types of forests under a subtropical microclimatic environment. Pedosphere, 24(2), 243–250.
Widén, B., & Majdi, H. (2001). Soil CO2 efflux and root respiration at three sites in a mixed pine and spruce forest: seasonal and diurnal variation. Canadian Journal of Forest Research, 31(5), 786–796.
Wofsy, S. C., Harriss, R. C., & Kaplan, W. A. (1988). Carbon dioxide in the atmosphere over the Amazon basin. Journal of Geophysical Research-Atmospheres, 93(D2), 1377–1387.
Xu, M., & Qi, Y. (2001). Soil-surface CO2 efflux and its spatial and temporal variations in a young ponderosa pine plantation in northern California. Global Change Biology, 7(6), 667–677.
Yuste, J. C., Janssens, I. A., Carrara, A., Meiresonne, L., & Ceulemans, R. (2003). Interactive effects of temperature and precipitation on soil respiration in a temperate maritime pine forest. Tree Physiology, 23, 1263–1270.
Zanchi, F. B., Waterloo, M. J., Kruijt, B., Kesselmeier, J., Luizão, F. J., Manzi, A. O., & Dolman, A. J. (2012). Soil CO2 efflux in central Amazonia: environmental and methodological effects. Acta Amazonica, 42(2), 173–184.
Zhang, L. H., Chen, Y. N., Zhao, R. F., & Li, W. H. (2010). Significance of temperature and soil water content on soil respiration in three desert ecosystems in Northwest China. Journal of Arid Environments, 74, 1200–1211.
Acknowledgments
The authors are grateful to Director, CSIR-National Botanical Research Institute, Lucknow, India, for providing necessary facilities and encouragement. The first author sincerely acknowledges the financial support by Department of Sciences and Technology, New Delhi, under Women Scientist Scheme-A. Dr. Nayan Sahu (CSIR-SRF), Dr. N. Manika (National PDF, SERB, DST), and Mr. Shiv Naresh Singh, SRF (Project), are also acknowledged for their assistance in the field measurements and helping in analyzing the data. Thanks are also due to PCCF (Wildlife), Government of Uttar Pradesh, Lucknow, and CCF cum Field Director (Dudhwa National Park), Bahraich, for granting permission to carry out the research and facilities to visit the area. All authors also sincerely thanks the two anonymous reviewers and Editors for their valuable comments and suggestions for improving the quality of the study.
Funding
This study was financially supported by the CSIR, New Delhi, under BSC-0109.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Terrestrial and Ocean Dynamics: India Perspective
Rights and permissions
About this article
Cite this article
Mishra, S., Chaudhary, L.B., Jain, M.K. et al. Interaction of abiotic factor on soil CO2 efflux in three forest communities in tropical deciduous forest from India. Environ Monit Assess 191 (Suppl 3), 796 (2019). https://doi.org/10.1007/s10661-019-7689-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7689-4