Abstract
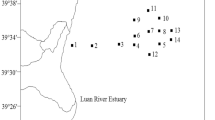
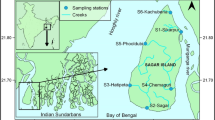
The present study was conducted during July 2013 (early phase of monsoon or EM) and September 2013 (later phase of monsoon or LM) to ascertain the intra-monsoonal variation on zooplankton, by selecting 15 study stations in the river Saptamukhi, one of the main estuaries in the Sundarbans Estuarine System (SES). In 2013, SES experienced an unusually high monsoonal rainfall also exacerbated by cloud burst event at Himalayan region (upper stretches of SES) which tremendously increased the river runoff. The present work was aimed to decipher the effect of this unusual precipitation during the monsoon season on zooplankton assemblages along with different hydrological parameters. The abundance of zooplankton was recorded as lower during EM compared to LM. Altogether, 56 zooplankton taxa were identified with copepods forming the predominant population. Thirty-three copepod species were reported with 25 calanoid species forming the bulk of the biomass followed by 5 and 3 species of cyclopoids and harpacticoid, respectively. A combination of multivariate cluster analysis, biotic indices, and canonical correspondence analysis revealed noticeable alterations in the zooplankton community structure across the spatio-temporal scale. Furthermore, significant intra-monsoonal changes in zooplankton population correlated with several hydrological parameters were clearly noticed. Paracalanus parvus, Bestiolina similis and Oithona similis were observed to be the most dominant copepod species in both sampling periods. The result of the present study provides new insight on estuarine zooplankton community after unusual rainfall during monsoon season, and provides further evidence to support the conservation and management of the SES ecosystem.









Similar content being viewed by others
References
Allen, S. K., Rastner, P., Arora, M., Huggle, C., & Stoffel, M. (2016). Lake outburst and debris flow disaster at Kedarnath, June 2013: hydrometeorological triggering and topographic predisposition. Landslides, 13, 1479–1491.
Alongi, D. M. (1990). The ecology of tropical soft-bottom benthic ecosystems. Oceanography and Marine Biology: An Annual Review, 28, 381–496.
Al-Yamani, F. Y., Skryabin, V., Gubanova, A., Khvorov, S., & Prusova, I. (2011). Marine zooplankton practical guide. Kuwait: Kuwait Institute for Scientific Research.
Beck, M. W., Heck, K., Able, K., Childers, D., Egglestone, D., Gillanders, B., et al. (2001). The identification, conservation and management of estuarine and marine nurseries for fish and invertebrates. Bioscience, 51, 633–641.
Bhattacharya, B. D., Hwang, J. S., Tseng, L. C., Sarkar, S. K., Rakshit, D., & Mitra, S. (2014a). Bioaccumulation of trace elements in dominant mesozooplankton group inhabiting in the coastal regions of Indian Sundarban mangrove wetland. Marine Pollution Bulletin, 87, 345–351.
Bhattacharya, B. D., Sarkar, S. K., & Bhattacharya, A. (2014b). Impact of the tropical cyclonic storm ‘Aila’ on the water quality and mesozooplankton of Sundarban mangrove coastal waters, India. Indian Journal of Geo-Marine Science, 43(2), 216–223.
Bhattacharya, B. D., Hwang, J. S., Sarkar, S. K., Rakshi, D., Murugan, K., & Li-Chun, T. (2015). Community structure of mesozooplankton in coastal waters of Sundarban mangrove wetland, India: a multivariate approach. Journal of Marine Systems, 141, 112–121.
Biswas, S. N., Nallamuthu, G., Sarangi, R. K., Bhattacharya, B. D., Sarkar, S. K., & Satpathy, K. K. (2013). Bloom of Hemidiscus hardmannianus (Bacillariophyceae) and its impact on water quality and plankton community structure in a mangrove wetland. Clean Soil Air Water, 41(4), 333–339.
Bollens, S. M., Breckenridge, J. K., Hooff, R. C., & Cordell, J. R. (2011). Mesozooplankton of the lower San Francisco Estuary: spatio-temporal patterns, ENSO effects and the prevalence of non-indigenous species. Journal of Plankton Research, 33(9), 1358–1377.
Campos, C. C., Garcia, T. M., Neumann-Leitao, S., & Soares, M. O. (2017). Ecological indicators and functional groups of copepod assemblages. Ecological Indicators, 83, 416–426.
Champalbert, G., & Pagano, M. (2002). Copepod feeding in a tuna fishery area of the tropical Atlantic Ocean. Comptes Rendus Biologies, 325(2), 171–177.
Chatterjee, M., Shankar, D., Sen, G. K., Sanyal, P., Sundar, D., Michael, G. S., et al. (2013). Tidal variations in the Sundarbans Estuarine System, India. Journal of Earth System Science, 122(4), 899–933.
Choudhury, A. K., & Bhadury, P. (2015). Relationship between N:P:Si ratio and phytoplankton community composition in a tropical estuarine mangrove ecosystem. Biogeosciences Discussions, 12, 2307–2355.
Chowdhury, C., Majumder, N., Ray, R., & Jana, T. K. (2012). Inter-annual abundance variation in some genera of diatom and zooplankton in a mangrove ecosystem. Biodiversity and Conservation, 21, 2029–2043.
Clarke, K. R., & Gorley, R. N. (2006). PRIMERv6: user manual/tutorial. Plymouth: PRIMER-E.
Clarke, K. R., & Warwick, R. M. (2001). Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory: PRIMER-E.
Clarke, K. R., Somerfield, P. J., & Gorley, R. N. (2008). Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage. Journal of Experimental Marine Biology and Ecology, 366, 56–69.
Conway, D. V. P., White, R. G., Hugues-Dit-Ciles, J., Gallienne, C. P., & Robins, D. B. (2003). Guide to the coastal and surface zooplankton of the south western Indian Ocean. Marine Biological Association of the United Kingdom, Occasional Publication. UKDEFRA.
Costello, J. H., Sullivan, B. K., & Gifford, D. J. (2006). A physical–biological interaction underlying variable phonological responses to climate change by coastal zooplankton. Journal of Plankton Research, 28(11), 1099–1105.
Das, P. K. (2013). ‘The Himalayan tsunami’- cloudburst, flash flood & death toll: a geographic post-mortem. IOSR Journal of Environmental Science, Toxicology and Food Technology, 7(2), 33–45.
David, V., Sautour, B., Chardy, P., & Leconte, M. (2005). Long-term changes of the zooplankton variability in a turbid environment: the Gironde estuary (France). Estuarine Coastal and Shelf Science, 64, 171–184.
Dhib, A., Brahim, M. B., Ziadi, B., Akrout, F., Turki, S., & Aleya, L. (2013). Factors driving the seasonal distribution of planktonic and epiphytic ciliates in a eutrophicated Mediterranean Lagoon. Marine Pollution Bulletin, 74, 383–395.
Dhib, A., Fertouna-Bellakhal, M., Turki, S., & Aleya, L. (2015). Harmful planktonic and epiphytic microalgae in a Mediterranean Lagoon: the contribution of the macrophyte Ruppia cirrhosa to microalgae dissemination. Harmful Algae, 45, 1–13.
Drira, Z., Hassen, M., Ayadi, H., Hamza, A., Zarrad, R., Bouain, A., & Aleya, L. (2010). Coupling of phytoplankton community structure to nutrients, ciliates and copepods in the Gulf of Gabes (South Ionian Sea, Tunisia). Journal of Marine Biological Association UK, 90, 1203–1215.
Giering, S. L. C., Sanders, R., Lampitt, R. S., Anderson, T. R., Tamburini, C. M., Boutrf, M. V., et al. (2014). Reconciliation of the carbon budget in the ocean’s twilight zone. Nature, 507, 480–483.
Grasshoff, K., Kremling, K., & Ehrhardt, M. (1999). Methods of seawater analysis (3rd ed.). Weinheim Germany: Verlag Chemie.
Harris, E. (1959). The nitrogen cycle in Long Island sound. Bulletin of Bingham Oceanographic Collection, 17, 31–42.
Islam, M. S., Hiroshi, U., & Masaru, T. (2005). Spatial distribution and trophic ecology of dominant copepods associated with turbidity maximum along the salinity gradient in a highly embayed estuarine system in Ariake Sea, Japan. Journal of Experimental Marine Biology and Ecology, 316, 101–115.
Jagadeesan, L., Jyothibabu, R., Anjushaa, A., Arya, P. M., Madhu, N. V., Muraleedharana, K. R., & Sudheesh, K. (2013). Ocean currents structuring the mesozooplankton in the Gulf of Mannar and the Palk Bay, southeast coast of India. Progress in Oceanography, 110, 27–48.
Kaizhi, L., Jianqiang, Y., Yehui, T., Liangmin, H., & Xingyu, S. (2014). Short-term variation in zooplankton community from Daya Bay with outbreaks of Penilia avirostris. Oceanologia, 56(3), 583–602.
Kasturirangan, L. R. (1963). In N. K. Pannikar (Ed.), A key for the identification of the more common planktonic copepoda of Indian coastal waters. New Delhi: Council of Scientific and Industrial Research.
Katechakis, A., Stibor, H., Sommer, U., & Hansen, T. (2004). Feeding selectivities and food niche separation of Acartia clausi, Penilia avirostris (Crustacea) and Doliolum denticulatum (Thaliacea) in Blanes Bay (Catalan Sea, NW Mediterranean). Journal of Plankton Research, 26, 589–603.
Kimmel, D. G., & Roman, M. R. (2004). Long-term trends in mesozooplankton abundance in Chesapeake Bay, USA: influence of freshwater input. Marine Ecology Progress Series, 267, 71–83.
Kimmerer, W. J. (2002). Effects of freshwater flow on abundance of estuarine organisms: physical effects or trophic linkages. Marine Ecology Progress Series, 243, 39–55.
Kotal, S. D., Roy, S. S., & Bhowmik, S. K. R. (2014). Catastrophic heavy rainfall episode over Uttarakhand during 16-18 June 2013- observational aspects. Current Science, 107(2), 237–245.
Kovach, W. L. (1998). MVSP–multi-Variate statistical package for windows, version 3.0. Pentraeth: Kovach computing services.
Krushkal, J. B., & Wish, M. (1978). Multidimensional scaling. Beverly Hills: Sage.
Lawrence, D., Valiela, I., & Tomasky, G. (2004). Estuarine calanoid copepod abundance in relation to season, salinity, and land-derived nitrogen loading, Waquoit Bay MA. Estuarine, Coastal and Shelf Science, 61, 547–557.
Lobry, J., Lepage, M., & Rochard, E. (2006). From seasonal patterns to a reference situation in an estuarine environment: example of the small fish and shrimp fauna of the Gironde estuary (SW France). Estuarine Coastal Shelf Science, 70, 239–250.
Lobry, J., David, V., Pasquaud, S., Lepage, M., Sautour, B., & Rochard, E. (2008). Diversity and stability of an estuarine trophic network. Marine Ecology Progress Series, 358, 13–25.
Longhurst, A., & Harrison, W. (1989). The biological pump: profiles of plankton production and consumption in the upper ocean. Progress in Oceanography, 22, 47–123.
Lough, R. G., & Mountain, D. G. (1996). Effect of small-scale turbulence on feeding rates of larvael cod and haddock in stratified water on Georges Bank. Deep Sea Research Part II: Topical Studies in Oceanography, 43, 1745–1772.
Mandal, B., Mukherjee, A., & Banerjee, S. (2013). A review on the ichthyofaunal diversity in mangrove based estuary of Sundarbans. Reviews in Fish Biology and Fisheries, 23, 365–374.
Margalef, D. R. (1957). Information theory in ecology. General Systems, 3, 36–71.
Margalef, R. (1967). Some concepts relative to the organization of plankton. Oceanography and Marine Biology: An Annual Review, 5, 257–289.
Marques, S. C., Azeiteiro, U. M., Leandro, S. M., Queiroga, H., Primo, A. L., Martinho, F., Viegas, I., & Pardal, M. A. (2008). Predicting zooplankton response to environmental changes in a temperate estuarine system. Marine Biology, 55, 531–541.
Mitra, A., Gastellani, C., Gentleman, W. C., Jonasdottir, S. H., Flynn, K. J., Bode, A., Halsband, C., et al. (2014). Bridging the gap between marine biogeochemical and fisheries sciences; configuring the zooplankton link. Progress in Oceanography, 129(B), 176–199.
Moderan, J., & Bouvais, P. (2010). Zooplankton community structure in a highly turbid environment (Charente estuary, France): spatio-temporal patterns and environmental control. Estuarine, Coastal and Shelf Science, 88, 219–232.
Mukhopadhyay, S. K., Biswas, H., De, T. K., & Jana, T. K. (2006). Fluxes of nutrients from the tropical river Hooghly at land-ocean boundary of Sundarbans NE coast of Bay of Bengal, India. Journal of Marine Systems, 62, 9–21.
Nair, V. R., & Peter, G. (1980). Zooplankton from the shelf waters off the west coast of India. Mahasagar, 13(1), 61–65.
Nakamura, Y., & Turner, J. T. (1997). Predation and respiration by the small cyclopoid copepod Oithona similis: how important is feeding on ciliates and heterotrophic flagellates? Journal of Plankton Research, 19, 1275–1288.
Nielsen, T. G., & Sabatini, M. (1996). Role of cyclopoid copepods Oithona sp. in North Sea plankton communities. Marine Ecology Progress Series, 139, 79–93.
Pages, F., Gonzalez, H. E., & Gonzalez, S. R. (1996). Diet of the gelatinous zooplankton in Hardangerfjord (Norway) and potential predatory impact by Aglantha digitale (Trachymedusae). Marine Ecology Progress Series, 139, 69–77.
Pielou, E. C. (1966). The measurement of diversity in different types of biological collections. Journal of Theoretical Biology, 13, 131–144.
Pielou, E. C. (1984). The interpretation of ecological data: a primer on classification and ordination. New York: Wiley.
Rakshit, D., Ganesh, P. S., & Sarkar, S. K. (2016). Choreotrich ciliate tintinnid (Protozoa: Ciliophora) in a tropical meso–macrotidal estuary, eastern part of India. Regional Studies in Marine Science, 3, 89–100.
Sarkar, S. K., & Bhattacharya, A. K. (2003). Conservation of biodiversity of the coastal resources of Sundarbans, Northeast India: an integrated approach through environmental education. Marine Pollution Bulletin, 47, 260–264.
Sarkar, S. K., Bhattacharya, B. D., & Saha, S. (2007). Spatial variation of zooplankton in Sundarban mangrove wetland, northeastern part of Bay of Bengal. ICFAI Journal of Life Science, 1, 7–21.
Saron, T., & Meitei, L. B. (2013). Seasonal variation of zooplankton population with reference to water quality of Iril River in Imphal. Current World Environment, 8(1), 133–141.
Selvin, P. J., & Aaron, P. L. (2015). Seasonal variation of zooplankton and pelagic fish catch in the fishing grounds off Tiruchendur coast, Gulf of Mannar, India. Ecohydrology & Hydrobiology, 15, 89–100.
Shannon, C. E. (1948). A mathematical theory of communication. The Bell System Technical Journal, 27, 379–423.
Simpson, E. H. (1949). Measurement of diversity. Nature, 163, 688.
Siokou-Frangou, I., Papathanassiou, E., Lepretre, A., & Frontier, S. (1998). Zooplankton assemblages and influence of environmental parameters on them in a Mediterranean coastal area. Journal of Plankton Research, 20, 847–870.
Sommer, U., & Stibor, H. (2002). Copepoda – Cladocera –Tunicata: The role of three major mesozooplankton groups in pelagic food webs. Ecological Research, 17(2), 161–174.
Stoecker, D. K., & Sanders, N. K. (1985). Differential grazing by Acartia tonsa on a dinoflagellate and a tintinnid. Journal of Plankton Research, 7, 85–100.
Strickland, J. D. H., & Parsons, T. R. (1972). A practical handbook of seawater analysis. Ottawa: Fishery Research Board of Canada.
Sun, J., Liu, D.-y., Jie, B., Gao, H.-w., & Han, X.-t. (2004). Phytoplankton community of the Bohai Sea in winter 2001. Journal of Ocean University of China, 34(3), 403–422.
Sundby, S., & Fossum, P. (1990). Feeding conditions of Arcto-Norwegian cod larvaee compared with the Rothschild-Osborn theory on small-scale turbulence and plankton contact rates. Journal of Plankton Research, 12, 1153–1162.
Thiel, R., Sepulveda, A., Kafemann, R., & Nellen, W. (1995). Environmental factors as forces structuring the fish community of the Elbe estuary. Journal of Fish Biology, 46, 47–69.
Thirunavukkarasu, K., Soundarapandian, P., Varadharajan, D., & Gunalan, B. (2013). Zooplankton composition and community structure of Kottakudi and Nari backwaters, South East of Tamil Nadu. Journal of Environmental and Analytical Toxicology, 4(1), 200.
Turner, J. T. (2004). The importance of small planktonic copepods and their roles in pelagic marine food webs. Zoological Studies, 43(2), 255–266.
Venkataramana, V., Sarma, V. V. S., & Reddy, A. M. (2017). Impact of river discharge on distribution of zooplankton biomass, community structure and food web dynamics in the Western coastal Bay of Bengal. Regional Studies of Marine Science., 16, 267–278. https://doi.org/10.1016/j.rsma.2017.09.007.
Wang, D., Jingjing, L., Pimao, C., & Yuan, M. (2014). Community characteristics and of zooplankton in Qinzhou Bay. Acta Ecologica Sinica, 34, 141–147.
Acknowledgements
The authors are grateful to The Vice Chancellor, Presidency University for her support and encouragement. Thanks are also due to Mr. Michael Holesworth for his comments to improve the manuscript. The authors gratefully acknowledge the Department of Forests and the Sundarbans Development Board, Government of West Bengal for the permission to work in some of the Reserve Forest areas. The authors thank anonymous reviewers for their constructive comments. Thanks are also due to all the members of SEP-2013 and Marine Ecology Laboratory for their overwhelming help and co-operation during the study.
Funding
The work was done as part of the Sundarbans Estuarine Program 2013, funded by INCOIS-MoES, Government of India to M.C. (Sanction No. F/INCOIS/HOOFS-05-2013 dated 21.06.2013) and MoES (MoES/36/OOIS/Extra/24/2013) and FRPDF grant of Presidency University to S.M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Nandy, T., Mandal, S. & Chatterjee, M. Intra-monsoonal variation of zooplankton population in the Sundarbans Estuarine System, India. Environ Monit Assess 190, 603 (2018). https://doi.org/10.1007/s10661-018-6969-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6969-8