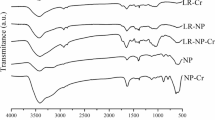
Abstract
A green and novel approach was demonstrated for successful remediation of arsenic from contaminated water by citric acid (CA) cross-linked water hyacinth root powder (RP). Different analytical techniques were used to investigate the binding and structural properties of prepared materials. Titanium dioxide played a significant role in the cross-linking process. Incorporation of CA into RP enhanced its integrity, and thus removal efficiency remained unaffected after several cyclic runs. Also the turbidity which formed due to treatment with uncross-linked RP was reduced to below the permissible limit. Effect of the amount of CA, material dose, treatment time, initial ion concentration, and pH were investigated. Use of 10% (w/w) CA was found to be sufficient to bring down the turbidity of the treated water below 2.5 nephelometric turbidity unit (NTU) without hampering the removal capacity/rate. A material dose of 5 g/L removed successfully total inorganic arsenic concentration to below 10 μg/L. The sorption process could be reasonably explained by Langmuir isotherm, and the maximum adsorption capacity was found to be 28 μg of arsenic/g. The material was found to be more efficient at acidic pH (pHZPC = 6.72). The sorption process was governed by a pseudo-second-order kinetic model.





Similar content being viewed by others
References
Bhatt, A. S., Sakaria, P. L., Vasudevan, M., Pawar, R. R., Sudheesh, N., Bajaj, H. C., et al. (2012). Adsorption of an anionic dye from aqueous medium by organoclays: equilibrium modeling, kinetic and thermodynamic exploration. RSC Advances, 2, 8663–8671.
Bonanno, G., & Lo Giudice, R. (2010). Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecological Indicators, 10, 639–645.
Bunluesin, S., Kruatrachue, M., Pokethitiyook, P., Upatham, S., & Lanza, G. R. (2007). Batch and continuous packed column studies of cadmium biosorption by Hydrilla verticillata biomass. Journal of Bioscience and Bioengineering, 103, 509–513.
Dutta, P. K., Ray, A. K., Sharma, V. K., & Millero, F. J. (2004). Adsorption of arsenate and arsenite on titanium dioxide suspensions. Journal of Colloid and Interface Science, 278, 270–275.
Elfeky, S. A., Imam, H., & Alsherbini, A. A. (2013). Bio-absorption of Ni and Cd on Eichhornia crassipes root thin film. Environmental Science Pollution Research, 20, 8220–8226.
Govindaswamy, S., Schupp, D. A., & Rock, S. A. (2011). Batch and continuous removal of arsenic using hyacinth roots. International Journal of Phytoremediation, 13, 513–527.
Hena, S. (2010). Removal of chromium hexavalent ion from aqueous solutions using biopolymer chitosan coated with poly 3-methyl thiophene polymer. Journal of Hazardous Material, 181, 474–479.
Ho, Y.-S. (2006). Isotherms for the sorption of lead onto peat: comparison of linear and non-linear methods. Polish Journal of Environmental Studies, 15, 81–86.
Hou, W., Chen, X., Song, G., Wang, Q., & Chang, C. C. (2007). Effects of copper and cadmium on heavy metal polluted water body restoration by duckweed (Lemna minor). Plant Physiology and Biochemistry, 45, 62–69.
Ibrahim, H. S., Ammar, N. S., Soylak, M., & Ibrahim, M. (2012). Removal of Cd (II) and Pb (II) from aqueous solution using dried water hyacinth as a biosorbent. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 96, 413–420.
Ibrahim, M., Mahani, R., Osman, O., & Scheytt, T. (2010). Effect of physical and chemical treatments on the electrical and structural properties of water hyacinth. The Open Spectroscopy Journal, 4, 32–40.
Ingole, N. W., & Bhole, A. G. (2003). Removal of heavy metals from aqueous solution by water hyacinth (Eichhornia crassipes). Journal of Water Supply: Research and Technology-AQUA, 52, 119–128.
Khan, A. A. (2006). Preparation, physico-chemical characterization, analytical applications and electrical conductivity measurement studies of an ‘organic–inorganic’composite cation-exchanger: polyaniline Sn (IV) phosphate. Reactive and Functional Polymers, 66, 1649–1663.
Kumar, M., & Puri, A. (2012). A review of permissible limits of drinking water. Indian Journal of Occupational and Environmental Medicine, 16, 40.
Laing, G. D., Tack, F. M. G., & Verloo, M. G. (2003). Performance of selected destruction methods for the determination of heavy metals in reed plants (Phragmites australis). Analytica Chimica Acta, 497, 191–198.
Menzel, C., Olsson, E., Plivelic, T. S., Andersson, R., Johansson, C., Kuktaite, R., et al. (2013). Molecular structure of citric acid cross-linked starch films. Carbohydrate Polymer, 96, 270–276.
Okeil, A. A. (2008). Citric acid crosslinking of cellulose using TiO2 catalyst by pad-dry-cure method. Polymer-Plastics Technology and Engineering, 47, 174–179.
Plazinski, W., & Rudzinski, W. (2009). Kinetics of adsorption at solid/solution interfaces controlled by Intraparticle diffusion: a theoretical analysis. The Journal of Physical Chemistry, 113, 12495–12501.
Reddya, N., & Yang, Y. (2010). Citric acid cross-linking of starch films. Food Chemistry, 118, 702–711.
Srivastava, M., Shrivastava, P., & D’Souza, S. S. F. (2011). Phytofiltration of arsenic from simulated contaminated water using Hydrilla verticillata in field conditions. Ecological Engineering, 37, 1937–1941.
Sekomo, C. B., Rousseau, D. P. L., Saleh, S. A., & Lens, P. N. L. (2012). Heavy metal removal in duckweed and algae ponds as a polishing step for textile wastewater treatment. Ecological Engineering, 44, 102–110.
Shaban, W., Rmalli, A., Harrington, C. F., Ayub, M., & Haris, P. I. (2005). A biomaterial based approach for arsenic removal from water. Journal of Environmental Monitoring, 7, 279–282.
Southichak, B., Nakano, K., Nomura, M., Chiba, N., & Nishimura, O. (2006). Phragmites australis: a novel biosorbent for the removal of heavy metals from aqueous solution. Water Research, 40, 2295–2302.
Srivastava, A., & Gopal, P. (2013). Efficiency of DHR as a biosorption of arsenic. International Research Journal of Environmental Sciences, 2, 71–76.
Srivastava, A., & Gopal, P. (2015). Kinetic study of arsenite adsorption onto dried hyacinth root powder. Research Journal of Chemical Sciences, 5, 41–48.
Takigawa, T., & Endo, Y. (2006). Effects of glutaraldehyde exposure on human health. Journal of Occupational Health, 48, 75–87.
Tiwari, S., Dixit, S., & Verma, N. (2007). An effective means of biofiltration of heavy metal contaminated water bodies using aquatic weed Eichhornia crassipes. Environmental Monitoring and Assessment, 129, 253–256.
Verma, V. K., Tewari, S., & Rai, J. P. N. (2008). Ion exchange during heavy metal bio-sorption from aqueous solution by dried biomass of macrophytes. Bioresource Technology, 99, 1932–1938.
Wang, S., Jin, X., Zhao, H., & Wu, F. (2008). Phosphate biosorption characteristics of a submerged macrophyte Hydrilla verticillata. Aquatic Botany, 89, 23–26.
Water, S. and World Health Organization, (2006). Guidelines for drinking-water quality [electronic resource]: incorporating first addendum. Vol. 1, Recommendations.
Wegelin, M., Gechter, D., Hug, S., Mahmud, A. & Motaleb, A. (2000). SORAS-a simple arsenic removal process. In WEDC CONFERENCE, 26, 255–258.
Win, D. T., Than, M. M., & Tun, S. (2003). Lead removal from industrial waters by water hyacinth. AU Journal of Technology, 6, 187–192.
Xu, Z., Li, Q., Gao, S., & Shang, J. K. (2010). As (III) removal by hydrous titanium dioxide prepared from one-step hydrolysis of aqueous TiCl4 solution. Water Research, 44, 5713–5721.
Xue, P.-Y., Li, G.-X., Liu, W.-J., & Yan, C.-Z. (2010). Copper uptake and translocation in a submerged aquatic plant Hydrilla verticillata (L.f.) Royle. Chemosphere, 81, 1098–1103.
Yang, C. Q., Wang, X., & Kang, I.-S. (1997). Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Research Journal, 67, 334–342.
Zheng, J.-C., Feng, H.-M., Lam, M. H.-W., Lam, P. K.-S., Ding, Y.-W., & Yu, H.-Q. (2009). Removal of Cu (II) in aqueous media by biosorption using water hyacinth roots as a biosorbent material. Journal of Hazardous Material, 171, 780–785.
Acknowledgments
Authors highly acknowledged DRL, Tezpur, for providing instrumental facilities in carrying out this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 33 kb)
Rights and permissions
About this article
Cite this article
Gogoi, P., Adhikari, P. & Maji, T.K. Bioremediation of arsenic from water with citric acid cross-linked water hyacinth (E. crassipes) root powder. Environ Monit Assess 189, 383 (2017). https://doi.org/10.1007/s10661-017-6068-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-6068-2