Abstract
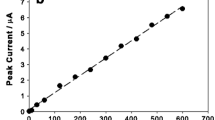
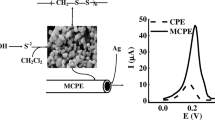
Amendment of a carbon paste electrode consisting of graphite and Nujol®, with a variety of organic and inorganic materials, allows direct adsorption of silver nanoparticles (AgNPs) from aqueous solution in either open or close circuit modes. The adsorbed AgNPs are detected by stripping voltammetry. Detection limits of less than 1 ppb Ag are achievable with a rotating disk system. More than one silver peak was apparent in many of the stripping voltammograms. The appearance of multiple peaks could be due to different species of silver formed upon stripping or variation in the state of aggregation or size of nanoparticles. With most of these packing materials, dissolved Ag+ was also extracted from aqueous solution, but, with a packing material made with Fe(II,III) oxide nanoparticles, only AgNPs were extracted. Therefore, it is the best candidate for determination of metallic AgNPs in aqueous environmental samples without interference from Ag+.












Similar content being viewed by others
References
Cheng, F., Betts, J. W., Kelly, S. M., Schaller, J., & Heinze, T. (2013). Synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using aminocellulose as a combined reducing and capping reagent. Green Chemistry, 15(4), 989–998.
Folsom, T. R., & Hodge, V. F. (1975). Experiments suggesting some first steps in the dispersal and disposal of plutonium and other alpha emitters in the ocean. Marine Sci. Comm., 13-4, 213–247.
Giovanni, M., & Pumera, M. (2012). Size dependant electrochemical behavior of silver nanoparticles with sizes of 10, 20, 40, 80 and 107 nm. Electroanalysis, 24(3), 615–617.
Huang, H., & Yang, X. (2004). Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydrate Research, 339, 2627–2631.
Hodge, V.F., Steinberg, S.M. (2012) Final report: the separation and analysis of manufactured nanomaterials in environmental samples. EPA Report on contract # EP-11-D-01618.
Jacobs, E. S. (1963). Anodic stripping voltammetry of gold and silver with carbon paste electrodes. Analytical Chemistry, 35(13), 2112–2115.
Kim, H., Ryoo, H., & Shin, K.-S. (2010). Adsorption and aggregation characteristics of silver nanoparticles onto a poly (4-vinylpyridine) film: a comparison with gold nanoparticles. Langmuir, 13, 10827–10832.
Nam, S.-W., Kim, S.-W., & An, Y.-J. (2013). No evidence for the genotoxic potential of gold, silver, zinc oxide, and titanium dioxide nanoparticles in the SOS chromotest. J. Applied toxicology, 33, 1061–1069.
Pace, H. E., Rogers, N. J., Jarolimek, C., Coleman, V. A., Gray, E. P., Higgins, C. P., & Ranville, J. F. (2012). Single particle inductively coupled plasma-mass spectrometry: a performance evaluation and method comparison in the determination of nanoparticle size. Environmental Science and Technology, 46(22), 12272–12280.
Pergantis, S. A., Jones-Lepp, T. L., & Heithmar, E. M. (2012). Hydrodynamic chromatography online with single particle-inductively coupled plasma mass spectrometry for ultra trace detection of metal-containing nanoparticles. Analytical Chemistry, 84(15), 6454–6462.
Saifuddin, N., Nian, C. Y., Zhan, L. W., & Ning, K. X. (2011). Chitosan-silver nanoparticles composite as a point-of-use drinking water filtration system for household to remove pesticides in water. Asian Journal of Biochemistry, 6(2), 142–159.
Stuart, E. J. E., Zhou, Y.-G., Rees, N. V., & Compton, R. G. (2012). Determining unknown concentrations of nanoparticles: the particle-impact electrochemistry of nickel and silver. RSC Advances, 2012(2), 6879–6884.
Stuart, E. J. E., Tschulik, K., Omanovic, D., Cullen, J. T., Jurkschat, K., Crossley, A., & Compton, R. G. (2013). Electrochemical detection of commercial silver nanoparticles: identification, sizing and detection in environmental media. Nanotechnology, 24, 444002 (6pp).
Toh, H. S., Batchelor-McAuley, C., Tschulik, K., Uhlemann, M., Crossley, A., & Compton, R. C. (2013). The anodic stripping voltammetry of nanoparticles: electrochemical evidence for the surface agglomeration of silver nanoparticles. Nanoscale, 5(11), 4884–4893.
USEPA (2014). 505-F-14-002 technical fact sheet—nanomaterials.
USEPA (2015) Control of nanoscale materials under the toxic substances control act, https://www.epa.gov. Accessed 15 Aug 2016.
Vance, M., Kulken, T., Vejerano, E. P., McGinnis, S. P., Hochell Jr., M. R., Rejeski, D., & Hull, M. (2015). Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein Journal of Nanotechnology, 6, 1769–1780.
Zhou, Y.-G., Rees, N. V., & Compton, R. G. (2011a). The electrochemical detection and characterization of silver nanoparticles in aqueous solution. Angewandte Chemie, International Edition, 50, 4219–4221.
Zhou, Y.-G., Rees, N. V., & Compton, R. G. (2011b). Electrode–nanoparticle collisions: the measurement of the sticking coefficient of silver nanoparticles on a glassy carbon electrode. Chemical Physics Letters, 514, 291–293.
Acknowledgements
The US Environmental Protection Agency through its Office of Research and Development funded, managed, and collaborated in the research described here under contract EP-11-D-1618 to the University of Nevada, Las Vegas. It has been subjected to agency review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steinberg, S., Hodge, V., Schumacher, B. et al. Sampling for silver nanoparticles in aqueous media using a rotating disk electrode: evidence for selective sampling of silver nanoparticles in the presence of ionic silver. Environ Monit Assess 189, 99 (2017). https://doi.org/10.1007/s10661-017-5809-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-5809-6