Abstract
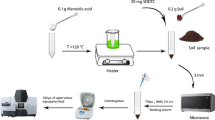
A solid-phase extraction method for separation and preconcentration of Ir(IV) ion by using activated carbon cloth (ACC) has been presented. Ir(IV) as their 1-(2-pyridylazo) 2-naphtol (PAN) chelate was adsorbed on ACC at pH 2.0 and was eluted from ACC with acidic dimethylformamide (DMF). The Ir(IV) concentration was determined at 536 nm as Ir(IV)–PAN complex by using UV–vis spectrophotometer. The analytical parameters including pH, sample and eluent flow rates, amount of PAN, eluent type, concentration, and sample volume were optimized. The effects of foreign ions on the recoveries of iridium were also investigated. The preconcentration factor was calculated as 60. The limit of detection (LOD) and the limit of quantification (LOQ) of the method were found as 0.039 and 0.129 μg L−1, respectively. The method was applied to soil and water samples for iridium determination.




Similar content being viewed by others
References
Afonin, M. V., Simanova, S. A., & Burmistrova, N. M. (2009). DEC extraction of iridium (III) and iridium (IV) chloride complexes by a new sorbent containing sulfur and nitrogen. Inorganic Materials, 45, 1543–1547.
Alam, M. Z., Ameem, E. S., Muyibi, S. A., & Kabbashi, N. A. (2009). The factors affecting the performance of activated carbon prepared from oil palm empty fruit bunches for adsorption of phenol. Chemical Engineering Journal, 155, 191–198.
Alothman, Z. A., Yilmaz, E., & Habila, M. (2015). Solid phase extraction of metal ions in environmental samples on 1-(2-pyridylazo)-2-naphthol impregnated activated carbon cloth. Ecotoxicology and Environmental Safety, 112, 74–79.
Ayranci, E., & Conway, B. E. (2001). Removal of phenol, phenoxide and chlorophenols from waste-waters by adsorption and electrosorption at high-area carbon felt electrodes. Journal of Electroanalytical Chemistry, 513, 100–108.
Bulut, V. N., Gundogdu, A., Duran, C., Senturk, H. B., Soylak, M., Elci, L., & Tufekci, M. (2007). A multi-element solid phase extraction method for trace metals in environmental samples on Amberlite XAD-2000. Journal of Hazardous Materials, 146, 155–163.
Dai, X., Chai, Z., Mao, X., Wang, J., Dong, S., & Li, K. (2000). An a-amino pyridine resin preconcentration method for iridium in environmental and geological samples. Analytica Chimica Acta, 403, 243–247.
Di, P., & Davey, D. E. (1995). On-line preconcentration and separation of palladium, platinum and iridium using a-amino pyridine resin with flame atomic absorption spectrometry. Talanta, 42, 685–692.
Duran, C., Ozdes, D., Sahin, D., Bulut, V. N., Gundogdu, A., & Soylak, M. (2011a). Preconcentration of Cd(II) and Cu(II) ions by coprecipitation without any carrier element in some food and water samples. Microchemical Journal, 98, 317–322.
Duran, A., Tuzen, M., & Soylak, M. (2011b). Speciation of Cr (III) and Cr (VI) in geological and water samples by ytterbium (III) hydroxide coprecipitation system and atomic absorption spectrometry. Food and Chemical Toxicology, 49, 1633–1637.
Elhami, S., & Sharifi, M. (2014). Development of an efficient procedure for the preconcentration of copper(II) after solid phase extraction on modified sawdust. Bulletin of Chemical Society of Ethiopia, 28, 321–328.
El-Sheikh, A. H., Sweileh, J. A., & Al-Degs, Y. S. (2007). Effect of dimensions of multi-walled carbon nanotubes on its enrichment efficiency of metal ions from environmental waters. Analytica Chimica Acta, 604, 119–126.
Ghaedi, M., Ahmadi, F., Tavakoli, Z., Montazerozohori, M., Khanmohammadi, A., & Soylak, M. (2008). Three modified activated carbons by different ligands for the solid phase extraction of copper and lead. Journal of Hazardous Materials, 152, 1248–1255.
Hong, H. J., Kim, H., Baek, K., & Yang, J. W. (2008). Removal of arsenate, chromate and ferricyanide by cationic surfactant modified powdered activated carbon. Desalination, 223, 221–228.
Karadas, C., & Kara, D. (2014). Determination of copper(II) in natural waters by extraction using n-o-vanillidine-2-amino-p-cresol and flame atomic absorption spectrometry. Instrumentation Science & Technology, 42, 548–561.
Li, L., Quinlivan, P. A., & Knappe, D. R. U. (2002). Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon, 40, 2085–2100.
Li, H. B., Du, Y. M., Wu, X. J., & Zhan, H. Y. (2004). Effect of molecular weight and degree of substitution of quaternary chitosan on its adsorption and flocculation properties for potential retention-aids in alkaline papermaking. Colloids and Surface A, 242, 1–8.
Makishima, A., Nakanishi, M., & Eizo, N. (2001). A group separation method for ruthenium, palladium, rhenium, osmium, iridium, and platinum using their bromo complexes and an anion exchange resin. Analytical Chemistry, 73, 5240–5246.
Masera, E., Mauchien, P., & Lerat, Y. (1996). Electrothermal atomization-laser induced fluorescence determination of iridium, rhodium, palladium, platinum and gold at the ng/l level in pure water. Spectrochimica Acta, 51B, 543–548.
Olorundare, O. F., Msagati, T. A. M., Krause, R. W. M., Okonkwo, J. O., & Mamba, B. B. (2015). Polyurethane composite adsorbent using solid phase extraction method for preconcentration of metal ion from aqueous solution. International Journal of Environmental Science and Technology, 12, 2389–2400.
Papaiconomou, N., Isabelle, B., & Eric, C. (2014). Extraction of iridium (IV) from aqueous solutions using hydrophilic/hydrophobic ionic liquids. RSC Advances, 89, 48260–48266.
Pirogov, A. V., & Havel, J. (1997). Determination of platinum, palladium, osmium, iridium, rhodium and gold as chloro complexes by capillary zone electrophoresis. Journal of Chromatography, 772A, 347–355.
Sahmetlioglu, E., Yilmaz, E., Aktas, E., & Soylak, M. (2014). Polypyrrole/multi-walled carbon nanotube composite for the solid phase extraction of lead(II) in water samples. Talanta, 119, 447–451.
Senturk, H. B., Gundogdu, A., Bulut, V. N., Duran, C., Soylak, M., Elci, L., & Tufekci, M. (2007). Separation and enrichment of gold(III) from environmental samples prior to its flame atomic absorption spectrometric determination. Journal of Hazardous Materials, 149, 317–323.
Shah, F., Soylak, M., Kazi, T. G., & Afridi, H. I. (2013). Preconcentration of lead from aqueous solution with activated carbon cloth prior to analysis by flame atomic absorption spectrometry: a multivariate study. Journal of Analytical Atomic Spectrometry, 28, 601–605.
Shanbhag, B., & Gosalia, R. (2008). Solvent extraction and spectrophotometric determination of Cu(II) with 3,4-diaminobenzophenone. Asian Journal of Chemistry, 20, 26–33.
Soleimani, M., Rafiei, B., & Siahpoosh, Z. H. (2015). Ghezeljeh montmorillonite nanoclay as a natural adsorbent for solid phase extraction of copper ions from food samples. Journal of Analytical Chemistry, 70, 794–803.
Soylak, M., & Akkaya, Y. (2002). An application of Diaion HP-20 solid phase extraction procedure for the separation of Cr(III), Cd(II) and Co(II) from urine. Trace Elements and Electrolytes, 19, 100–104.
Soylak, M., Elci, L., & Dogan, M. (1995). Preconcentration of trace amounts of tungsten on Amberlite XAD-7 for its spectrophotometric determination in hot spring water. Fresenius Journal of Analytical Chemistry, 351, 308–310.
Soylak, M., Elci, L., & Dogan, M. (1997). Determination of molybdenum in water and geological samples from Sorgun-Yozgat Region after separation and preconcentration: comparative results. Kuwait Journal of Science and Engineering, 24, 87–91.
Soylak, M., Divrikli, U., Elci, L., & Dogan, M. (1998). Column chromatographic preconcentration and enrichment of copper, nickel and iron in ammonium salts by graphite furnace atomic absorption spectrometry. Kuwait Journal of Science and Engineering, 25, 387–395.
Soylak, M., Uzun, A., & Elci, L. (2002). Sorbent extraction of copper, lead, nickel and cadmium ions in dialysis concentrates before their atomic absorption spectrometric determinations. Trace Elements and Electrolytes, 19, 15–19.
Soylak, M., Akkaya, Y., & Elci, L. (2003). Application of the solid phase extraction procedure on chromatographic mini column filled with Diaion HP-20 for determination of copper, cobalt, cadmium, iron and manganese in textile products and textile wastewaters. Trace Elements and Electrolytes, 20, 16–22.
Soylak, M., Saracoglu, S., & Elci, L. (2004). Investigation of adsorption of metal ions on polystyrene divinyl benzene copolymers by scanning electron microscopy and flame atomic absorption spectrometry. Asian Journal of Chemistry, 16, 1673–1680.
Sun, P. P., & Lee, M. S. (2011). Separation of Ir(IV) and Rh(III) from mixed chloride solutions by solvent extraction. Hydrometallurgy, 105, 334–340.
Svancara, I., Galık, M., & Vytras, K. (2007). Stripping voltammetric determination of platinum metals at a carbon paste electrode modified with cationic surfactants. Talanta, 72, 512–518.
Taher, M. A. (2003). Flame atomic absorption spectrometric determination of trace lead after solid-liquid extraction and preconcentration using 1-(2-pyridylazo)-2-naphthol. Croatica Chemica Acta, 76, 273–277.
Taher, M. A., Puri, S., Bansal, R. K., & Puri, B. K. (1997). Derivative spectrophotometric determination of iridium after preconcentration of its 1-(2-pyridylazo)-2-naphthol complex on microcrystalline naphthalene. Talanta, 45, 411–416.
Terzyk, A. P. (2004). Molecular properties and intermolecular forces—factors balancing the effect of carbon surface chemistry in adsorption of organics from dilute aqueous solutions. Journal of Colloid and Interface Science, 275, 9–29.
Zeinali, N., Ghaedi, M., & Shafie, J. (2014). Competitive adsorption of methylene blue and brilliant green onto graphite oxide nano particle following: derivative spectrophotometric and principal component-artificial neural network model methods for their simultaneous determination. Journal of Industrial and Engineering Chemistry, 20, 3550–3558.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozkantar, N., Yilmaz, E., Soylak, M. et al. Solid-phase extraction of iridium from soil and water samples by using activated carbon cloth prior to its spectrophotometric determination. Environ Monit Assess 187, 501 (2015). https://doi.org/10.1007/s10661-015-4720-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4720-2