Abstract
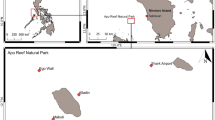
Coral reef ecosystems fringing the coastline of Dahab (South Sinai, Egypt) have experienced increasing anthropogenic disturbance as an emergent international tourism destination. Previous reports covering tourism-related impacts on coastal environments, particularly mechanical damage and destructive fishing, have highlighted the vital necessity for regular ecosystem monitoring of coral reefs near Dahab. However, a continuous scientific monitoring programme of permanent survey sites has not been established to date. Thus, this study conducted in situ monitoring surveys to investigate spatio-temporal variability of benthic reef communities and selected reef-associated herbivores along with reef health indicator organisms by revisiting three of the locally most frequented dive sites during expeditions in March 2010, September 2011 and February 2013. In addition, inorganic nutrient concentrations in reef-surrounding waters were determined to evaluate bottom-up effects of key environmental parameters on benthic reef community shifts in relation to grazer-induced top-down control. Findings revealed that from 2010 to 2013, live hard coral cover declined significantly by 12 % at the current-sheltered site Three Pools (TP), while showing negative trends for the Blue Hole (BH) and Lighthouse (LH) sites. Hard coral cover decline was significantly and highly correlated to a substantial increase in turf algae cover (up to 57 % at TP) at all sites, replacing hard corals as dominant benthic space occupiers in 2013. These changes were correlated to ambient phosphate and ammonium concentrations that exhibited highest values (0.64 ± 0.07 μmol PO4 3− l−1, 1.05 ± 0.07 μmol NH4 + l−1) at the degraded site TP. While macroalgae appeared to respond to both bottom-up and top-down factors, change in turf algae was consistent with expected indications for bottom-up control. Temporal variability measured in herbivorous reef fish stocks reflected seasonal impacts by local fisheries, with concomitant changes in macroalgal cover. These findings represent the first record of rapid, localised change in benthic reef communities near Dahab, consistent with indications for bottom-up controlled early-stage phase shifts, underlining the necessity for efficient regional wastewater management for coastal facilities.



Similar content being viewed by others
References
Alvarez-Filip, L., Dulvy, N. K., Gill, J. A., Cote, I. M., & Watkinson, A. R. (2009). Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proceedings of the Royal Society B: Biological Sciences, 276, 3019–3025.
Ammar, M. S. A., Bouwmeester, J., Riegl, B., Hausser, J., & Keck, A. (2006). Possible causes, consequences of changes and future of coral reefs in Dahab, Gulf of Aqaba, Red Sea, Egypt. Egyptian Journal of Aquatic Research, 32, 160–179.
Ammar, M. S. A. (2009). Assessment of present status and future needs of four coral reef sites along the Gulf of Aqaba, Egypt. The Open Environmental Pollution and Toxicology Journal, 1, 34–42.
Barott, K., & Rohwer, F. (2012). Unseen players shape benthic competition on coral reefs. Trends in Microbiology, 20, 621–628.
Bellwood, D. R., & Fulton, C. J. (2008). Sediment-mediated suppression of herbivory on coral reefs: decreasing resilience to rising sea-levels and climate change? Limnology and Oceanography, 53, 2695–2701.
Cantin, N. E., Cohen, A. L., Karnauskas, K. B., Tarrant, A. M., & McCorkle, D. C. (2010). Ocean warming slows coral growth in the central Red Sea. Science, 329, 322–325.
CAPMAS (2014). Egypt in figures 2013. Tourism (pp. 155). Central Agency for Public Mobilization and Statistics: Arab Republic of Egypt.
Cesar, H. (2003). Report on the economic valuation of the Egyptian Red Sea coral reef. Monitoring, Verification, and Evaluation (MVE) Unit of the Egyptian Environmental Policy Program.
EcoConServ. (2005). Study on status of the environment and relevant policies / measures in Egypt. Environmental Solutions (p. 70). Japan: Overseas Environmental Cooperation.
English, S., Wilkinson, C., & Baker, V. (1997). Survey manual for tropical marine resources. Townsville, Australia: Australian Institute of Marine Science.
Fabricius, K. E., Genin, A., & Benayahu, Y. (1995). Flow-dependent herbivory and growth in zooxanthellae-free soft corals. Limnology and Oceanography, 40, 1290–1301.
Fine, M., Gildor, H., & Genin, A. (2013). A coral reef refuge in the Red Sea. Global Change Biology, 19, 3640–3647.
Fowler, A. J. (1987). The development of sampling strategies for population studies of coral reef fishes. A case study. Coral Reefs, 6, 49–58.
Gardner, T. A., Côté, I. M., Gill, J. A., Grant, A., & Watkinson, A. R. (2003). Long-term region-wide declines in Caribbean corals. Science, 301, 958–960.
Genin, A., Lazar, B., & Brenner, S. (1995). Vertical mixing and coral death in the Red Sea following the eruption of Mount Pinatubo. Nature, 377, 507–510.
Haas, A., el-Zibdah, M., & Wild, C. (2010). Seasonal monitoring of coral-algae interactions in fringing reefs of the Gulf of Aqaba, Northern Red Sea. Coral Reefs, 29, 93–103.
Haas, A. F., Nelson, C. E., Wegley Kelly, L., Carlson, C. A., Rohwer, F., et al. (2011). Effects of coral reef benthic primary producers on dissolved organic carbon and microbial activity. PLoS ONE, 6(11), e27973.
Haas, A. F., Gregg, A. K., Smith, J. E., Abieri, M. L., Hatay, M., & Rohwer, F. (2013). Visualization of oxygen distribution patterns caused by coral and algae. PeerJ. doi:10.7717/peerj.106.
Hannak, J. S. (2008). A snorkel trail based on reef condition and visitor perception as a management tool for a threatened shallow water reef in Dahab (South Sinai, Egypt). MSc thesis (pp. 54), Universität Wien.
Hannak, J. S., Kompatscher, S., Stachowitsch, M., & Herler, J. (2011). Snorkelling and trampling in shallow-water fringing reefs: risk assessment and proposed management strategy. Journal of Environmental Management, 92, 2723–2733.
Hasler, H., & Ott, J. A. (2008). Diving down the reefs? Intensive diving tourism threatens the reefs of the northern Red Sea. Marine Pollution Bulletin, 56, 1788–1794.
Hawkins, J. P., & Roberts, C. M. (1994). The growth of coastal tourism in the Red Sea: present and future effects on coral reefs. Ambio, 23(8), 503–508.
Hawkins, J. P. & Roberts, C. M. (1997). Estimating the carrying capacity of coral reefs for SCUBA diving. Proceedings of the 8th Coral Reef Symposium, 1923–1926.
Hodgson, G. (1990). Sediment and the settlement of larvae of the reef coral Pocillopora damicornis. Coral Reefs, 9, 41–43.
Hodgson, G. (1996). Sedimentation damage to reef corals. Biological Conservation, 76, 217–218.
Hodgson, G., Maun, L., & Shuman, C. (2006). Reef Check Survey Manual. Reef Check. Institute of the Environment. Los Angeles, CA: University of California.
Hoegh-Guldberg, O., Mumby, P. J., Hooten, A. J., Steneck, R. S., Greenfield, P., Gomez, E., Harvell, C. D., Sale, P. F., Edwards, A. J., Caldeira, K., Knowlton, N., Eakin, C. M., Iglesias-Prieto, R., Muthiga, N., Bradbury, R. H., Dubi, A., & Hatziolos, M. E. (2007). Coral reefs under rapid climate change and ocean acidification. Science, 318, 1737–1742.
Holmes, R. M., Aminot, A., Kérouel, R., Hooker, B., & Peterson, B. J. (1999). A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Canadian Journal of Fisheries and Aquatic Sciences, 56, 1801–1808.
Hughes, T. P., Keller, B. D., Jackson, J. B. C., & Boylem, M. J. (1985). Mass mortality of the echinoid Diadema antillarum Philippi in Jamaica. Bulletin of Marine Science, 36, 377–384.
Hughes, T. P. (1989). Community structure and diversity of coral reefs: The role of history. Ecology, 70, 275–279.
Hughes, T. P. (1994). Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science, 265, 1547–1551.
Hughes, T. P., Baird, A. H., Bellwood, D. R., Card, M., Connolly, S. R., Folke, C., Grosberg, R., Hoegh-Guldberg, O., Jackson, J. B. C., & Kleypas, J. (2003). Climate change, human impacts, and the resilience of coral reefs. Science, 301, 929–933.
Hughes, T. P., Graham, N. A. J., Jackson, J. B. C., Mumby, P. J., & Steneck, R. S. (2010). Rising to the challenge of sustaining coral reef resilience. Trends in Ecology and Evolution, 25(11), 633–642.
Jameson, S. C., Ammar, M. S. A., Saadalla, E., Mostafa, H. M., & Riegl, B. (1999). A coral damage index and its application to diving sites in the Egyptian Red Sea. Coral Reefs, 18, 333–339.
Jobbins, G. (2006). Tourism and coral-reef based conservation: can they coexist? In I. M. Cote & J. D. Reynolds (Eds.), Coral Reef Conservation (pp. 237–263). Cambridge: Cambridge University Press.
Jompa, J., & McCook, L. J. (2003). Coral–algal competition: macroalgae with different properties have different effects on corals. Marine Ecology Progress Series, 258, 87–95.
Jouffray, J.-B., Nyström, M., Norström, A., Williams, I., Wedding, L., Kittinger, J.N., & Williams, G.J., (2014). Identifying multiple coral reef regimes and their drivers across the Hawaiian Archipelago. Philosophical Transactions of the Royal Society B: Biological Sciences, 370, doi:10.1098/rstb.2013.0268.
Kline, D. I., Kuntz, N. M., Breitbart, M., Knowlton, N., & Rohwer, F. (2006). Role of elevated organic carbon levels and microbial activity in coral mortality. Marine Ecology Progress Series, 314, 119–125.
Kotb, M. M. A., Hanafy, M. H., Rirachche, H., Matsumura, S., Al-Sofyani, A. A., Ahmed, A. G., Bawazir, G., & Al-Horani, F. A. (2008). Status of coral reefs in the Red Sea and Gulf of Aden region. In C. Wilkinson (Ed.), Status of coral reefs of the world: 2008. Townsville, Australia: Global Coral Reef Monitoring Network and Reef and Rainforest Research Center.
Kremien, M., Shavit, U., Mass, T., & Genin, A. (2013). Benefit of pulsation in soft corals. Proceedings of the National Academy of Sciences, 110(22), 8978–8983.
Littler, M. M., Littler, D. S., & Brooks, B. L. (2006). Harmful algae on tropical coral reefs: Bottom-up eutrophication and top-down herbivory. Harmful Algae, 5, 565–585.
Loya, Y. (1978). Plotless and transect methods. In D. R. Stoddart & R. E. Johannes (Eds.), Coral reefs: research methods (pp. 197–217). Paris: United Nations Educational, Scientific and Cultural Organization.
Loya, Y. (2004). The coral reefs of Eilat—past, present and future: three decades of coral community structure studies. In E. Rosenberg & Y. Loya (Eds.), Coral reef health and disease (p. 29). Berlin: Springer-Verlag.
Mabrouk, A. (2007). Management Plan for NMRPA. Nature Conservation Sector, NCS–UNEP.
McClanahan, T. R., & Karnauskas, M. (2010). Relationships between benthic cover, current strength, herbivory, and a fisheries closure in Glovers Reef Atoll, Belize. Coral Reefs, 30, 9–19.
McManus, J. W., & Polsenberg, J. F. (2004). Coral-algal phase shifts on coral reefs: ecological and environmental aspects. Progress in Oceanography, 60, 263–279.
Mumby, P. J. (2006). The impact of exploiting grazers (Scaridae) on the dynamics of Caribbean coral reefs. Ecological Applications, 16, 747–769.
Murphy, J., & Riley, J. (1962). A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta, 27, 31–36.
Nadon, M. O., & Stirling, G. (2006). Field and simulation analyses of visual methods for sampling coral cover. Coral Reefs, 25, 177–185.
Naumann, M. S., Haas, A., Struck, U., Mayr, C., el-Zibdah, M., & Wild, C. (2010). Organic matter release by the dominant hermatypic corals of the Northern Red Sea. Coral Reefs, 29, 649–660.
Naumann, M. S., Richter, C., Mott, C., El-Zibdah, M., Manasrah, R., & Wild, C. (2012). Budget of coral-derived organic carbon in a fringing coral reef of the Gulf of Aqaba, Red Sea. Journal of Marine Systems, 105, 20–29.
Naumann, M. S., Jantzen, C., Haas, A. F., Iglesias-Prieto, R., & Wild, C. (2013). Benthic primary production budget of a Caribbean reef lagoon (Puerto Morelos, Mexico). PLoS ONE, 8(12), e82923.
Nugues, M. M., Smith, G. W., van Hooidonk, R. J., Seabra, M. I., & Bak, R. P. M. (2004). Algal contact as a trigger for coral disease. Ecology Letters, 7, 919–923.
OECD (2006). African economic outlook 2005–2006 (pp. 252). Egypt.
PERSGA (2001). Strategic Action Programme for the Red Sea and Gulf of Aden–Country Reports. Jeddah, PERSGA. The Regional Organization for the Conservation of the Environment of the Red Sea and Gulf of Aden.
Rasheed, M., Badran, M. I., Richter, C., & Huettel, M. (2002). Effect of reef framework and bottom sediment on nutrient enrichment in a coral reef of the Gulf of Aqaba, Red Sea. Marine Ecology Progress Series, 239, 277–285.
ReefBase database (2013) ReefGIS online services, http://www.reefgis.reefbase.org.
Riegl, B., & Velimirov, B. (1991). How many damaged corals in Red Sea reef systems? A quantitative survey. Hydrobiologia, 216(217), 249–256.
Rinkevich, B. (2005). What do we know about Eilat (Red Sea) reef degradation? A critical examination of the published literature. Journal of Experimental Marine Biology and Ecology, 327, 183–200.
Roff, G., & Mumby, P. J. (2012). Global disparity in the resilience of coral reefs. Trends in Ecology & Evolution, 27(7), 404–413.
Rogers, C. S. (1990). Responses of coral reefs and reef organisms to sedimentation. Marine Ecology Progress Series, 62, 185–202.
Russ, G. R. (2003). Grazer biomass correlates more strongly with production than with biomass of algal turfs on a coral reef. Coral Reefs, 22, 63–67.
Salem, M. (1999). Management of fishing in the Ras Mohammed National Park with special reference to the fishery for Lethrinus nebulosus (Forskal, 1775). PhD thesis, University of York, UK.
Samy, M., Sánchez Lizaso, J. L., & Forcada, A. (2011). Status of marine protected areas in Egypt. Animal Biodiversity and Conservation, 34, 165–177.
SEAM (2004) Growth in tourism in South Sinai: challenges facing tourism development. Department for International Development, UK, Support for Environmental Assessment and Management Programme.
SEAM (2004) South Sinai Governorate Environmental Action Plan (GEAP). Department for International Development, UK, Support for Environmental Assessment and Management Programme.
Sheppard, C. R. C. (1982). Coral populations on reef slopes and their major controls. Marine Ecology Progress Series, 7, 83–115.
Smith, S. V. (1984). Phosphorus versus nitrogen limitation in the marine environment. Limnology and Oceanography, 29, 1149–1160.
Smith, J. E., Smith, C. M., & Hunter, C. L. (2001). An experimental analysis of the effects of herbivory and nutrient enrichment on benthic community dynamics on a Hawaiian reef. Coral Reefs, 19, 332–342.
Smith, J. E., Shaw, M., Edwards, R. A., Obura, D., Pantos, O., Sala, E., Sandin, S. A., Smriga, S., Hatay, M., & Rohwer, F. L. (2006). Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecology Letters, 9, 835–845.
Strickland, J., & Parsons, T. (1968). A practical handbook of seawater analysis. Ottawa: Fisheries Research Board of Canada, Bulletin 167.
Sweatman, H., Cheal, A., Coleman, G., Emslie, M., Johns, K., Jonker, M., Miller, I., & Osborne, K. (2008). Long-term monitoring of the Great Barrier Reef. Townsville, Australia: Australian Institute of Marine Science.
Tanner, J. E. (1995). Competition between scleractinian corals and macroalgae: an experimental investigation of coral growth, survival and reproduction. Journal of Experimental Marine Biology and Ecology, 190, 151–168.
Tilot, V., Leujak, W., Ormond, R. F. G., Ashworth, J. A., & Mabrouk, A. (2008). Monitoring of South Sinai coral reefs: influence of natural and anthropogenic factors. Aquatic Conservation: Marine And Freshwater Ecoystems, 18, 1109–1126.
Van Den Hoek, C., Breeman, A. M., Bak, R. P. M., & Van Buurt, G. (1978). The distribution of algae, corals and gorgonians in relation to depth, light attenuation, water movement and grazing pressure in the fringing coral reef of Curacao, Netherlands Antilles. Aquatic Botany, 5, 1–46.
Wielgus, J., Chadwick-Furman, N. E., & Dubinsky, Z. (2004). Coral cover and partial mortality on anthropogenically impacted coral reefs at Eilat, northern Red Sea. Marine Pollution Bulletin, 48, 248–253.
Wild, C., Niggl, W., Naumann, M. S., & Haas, A. F. (2010). Organic matter release by Red Sea coral reef organisms—potential effects on microbial activity and in-situ O2 availability. Marine Ecology Progress Series, 411, 61–71.
Wild, C., Hoegh-Guldberg, O., Naumann, M. S., Colombo-Pallotta, M. F., Ateweberhan, M., Fitt, W. K., Iglesias-Prieto, R., Palmer, C., Bythell, J. C., Ortiz, J. C., Loya, Y., & van Woesik, R. (2011). Climate change impedes scleractinian corals as primary reef ecosystem engineers. Marine and Freshwater Research, 62, 205–215.
Wild, C., & Naumann, M. S. (2013). Effect of active water movement on energy and nutrient acquisition in coral reef-associated benthic organisms. Proceedings of the National Academy of Sciences, 110(22), 8767–8768.
Wilkinson, C. (2004). Status of coral reefs of the world: 2004. Townsville, Australia: Global Coral Reef Monitoring Network and Reef and Rainforest Research Center.
Wilkinson, C. (2008). Status of coral reefs of the world: 2008. Townsville, Australia: Global Coral Reef Monitoring Network and Reef and Rainforest Research Center.
Wilson, S. K., Graham, N. A. J., Prachett, M. S., Jones, G. P., & Polunin, N. V. C. (2006). Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Global Change Biology, 12, 2220–2234.
Wolf-Vecht, A., Paldora, N., & Brenner, S. (1992). Hydrographic indications of advection/convection effects in the Gulf of Eilat. Deep-Sea Research, 39, 1393–1401.
Acknowledgments
We want to thank A. Haas, C. Haacke, A. Gabrenya, C. Williamson, K. Korczyk, the student participants of the 2011 and 2013 excursions, C. Alter and L. Wagenknecht for their support on site. We are also grateful to two anonymous reviewers and topic editor A. Elvir for helpful comments. This study was supported by grant Wi 2677/6-1 of the German Research Foundation (DFG) and the Leibniz Association.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naumann, M.S., Bednarz, V.N., Ferse, S.C.A. et al. Monitoring of coastal coral reefs near Dahab (Gulf of Aqaba, Red Sea) indicates local eutrophication as potential cause for change in benthic communities. Environ Monit Assess 187, 44 (2015). https://doi.org/10.1007/s10661-014-4257-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4257-9