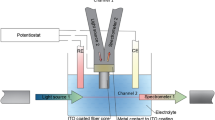
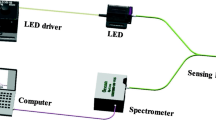
Abstract
A phenosafranine-containing Nafion film attached to the distal end of a fiber-optic probe forms a functional redox-sensitive optical sensor. The synthetic cationic photoactive dye phenosafranine, 3,7-diamino-5-phenylphenazinium chloride, responds with changes in light absorbance between its oxidized and reduced forms. This optical property persists when phenosafranine is sorbed into Nafion, a perfluorosulfonate anionic film. Optical properties of the sensor are similar to those seen by others in solution. At high redox conditions, such as an open nitrogen-purged aqueous pH 6.5 solution, optical absorbance of phenosafranine is high, while at low redox conditions, such as an aqueous pH 6.5 iron(II) solution, optical absorbance of phenosafranine is low. Titration of a closed pH 6.5 aqueous solution with a standard iron(II) solution lowers redox potential in a predictable manner and can be followed by the optical redox sensor in parallel with a commercial redox potential electrode.





Similar content being viewed by others
References
Atteia, O., & Guillot, C. (2007). Factors controlling BTEX and chlorinated solvents plume length under natural attenuation conditions. Journal of Contaminant Hydrology, 90, 81–104. doi:10.1016/j.jconhyd.2006.09.012.
Bakker, E., & Qin, Y. (2006). Electrochemical sensors. Analytical Chemistry, 78, 3965–3983. doi:10.1021/ac060637m.
Barbieri, M., Carrera, J., Sanchez-Vila, X., Ayora, C., Cama, J., Köck-Schulmeyer, M., et al. (2011). Microcosm experiments to control anaerobic redox conditions when studying the fate of organic micropollutants in aquifer material. Journal of Contaminant Hydrology, 126, 330–345. doi:10.1016/j.jconhyd.2011.09.003.
Borch, T., Kretzschmar, R., Kappler, A., van Cappellen, P., Ginder-Vogel, M., Voegelin, A., et al. (2010). Biogeochemical redox processes and their impact on contaminant dynamics. Environmental Science and Technology, 44, 15–23. doi:10.1021/es9026248.
Bowler, P. G., Duerben, B. I., & Armstrong, D. G. (2001). Wound microbiology and associated approaches to wound management. Clinical Microbiology Reviews, 14(2), 244–269.
Christensen, T. H., Bjerg, P. L., Banwart, S. A., Jakobsen, R., Heron, G., & Albrechtsen, H. (2000). Characterization of redox conditions in groundwater contaminant plumes. Journal of Contaminant Hydrology, 45, 165–241. doi:10.1016/S0169-7722(00)00109-1.
Chudyk, W. (1989). Fiber optic methods for volatile organic compounds in groundwater. In N. M. Ram, R. F. Christman, & K. P. Cantor (Eds.), Significance and treatment of volatile organic compounds in water supplies (pp. 87–99). Chelsea: Lewis.
Feidler, S., Vepraskas, M., & Richardson, J. (2007). Soil redox potential: importance, field measurements, and observations. Advances in Agronomy, 94, 1–54. doi:10.1016/S0065-2113(06)94001-2.
Gopidas, K. R., & Kamat, P. V. (1991). Photochemistry in polymers. Photoinduced electron transfer between phenosafranine and triethylamine in perfluorosulfonate membrane. Journal of Physical Chemistry, 94, 4726–4727. doi:10.1021/j100374a064.
Hinchey, E. K., & Schaffner, L. C. (2005). An evaluation of electrode insertion techniques for measurement of redox potential in estuarine sediments. Chemosphere, 59, 703–710. doi:10.1016/j.chemosphere.2004.10.029.
Jones, B. D., & Ingle, J. D., Jr. (2005). Evaluation of redox indicators for determining sulfate-reducing and dechlorinating conditions. Water Research, 39, 4343–4354. doi:10.1016/j.watres.2005.09.006.
Kierat, R. M., Thaler, B. M. B., & Krämer, R. (2010). A fluorescent redox sensor with tuneable oxidation potential. Bioorganica Medical Chemistry Letter, 20, 1457–1459. doi:10.1016/j.bmcl.2009.03.171.
Komura, T., Niu, G. Y., Yamaguchi, T., & Asano, M. (2003). Redox and ionic-binding switched fluorescence of phenosafranine and thionine included in Nafion films. Electrochimica Acta, 48, 631–639. doi:10.1016/S0013-4686(02)00733-8.
Lemmon, T. L., Westall, J. C., & Ingle, J. D., Jr. (1996). Development of redox sensors for environmental applications based on immobilized redox indicators. Analytische Chemie, 68, 947–953. doi:10.1021/ac950885a.
Maineult, A., Bernabé, Y., & Ackerer, P. (2006). Detection of advected, reacting redox fronts from self-potential measurements. Journal of Contaminant Hydrology, 86, 32–52. doi:10.1016/j.jconhyd.2006.02.007.
Masscheleyn, P. H., Delaune, R. D., & Patrick, W. H., Jr. (1991). Effect of redox potential and ph on arsenic speciation and solubility in a contaminated soil. Environmental Science and Technology, 25, 1414–1419. doi:10.1021/es00020a008.
Miehr, R., Tratnyek, P. G., Bandstra, J. Z., Scherer, M. M., Alowitz, M. J., & Bylaska, E. J. (2004). Diversity of contaminant reduction reactions by zerovalent iron: role of the reductate. Environmental Science and Technology, 38, 139–147. doi:10.1021/es034237h.
Newcombe, D. T., Cardwell, T. J., Cattrall, R. W., & Kolev, S. D. (1999). An optical redox chemical sensor based on ferroin immobilised in a Nafion® membrane. Analytica Chimica Acta, 401, 137–144. doi:10.1016/S0003-2670(99)00474-2.
Newcombe, D. T., Cardwell, T. J., Cattrall, R. W., & Kolev, S. D. (2000). An optical membrane redox chemical sensor for the determination of ascorbic acid. Laboratory Rob. Automated, 12(4), 200–204. doi:10.1002/1098-2728(2000)12:4<200::AID-LRA5>3.0.CO;2–2.
Owens, P. R., Wilding, L. P., Lee, L. M., & Herbert, B. E. (2005). Evaluation of platinum electrodes and three electrode potential standards to determine electrode quality. Soil Science Society American Journal, 69, 1541–1550. doi:10.2136/sssaj2003.0205.
Passos, M. L. C., Saraiva, M. L. M. F. S., & Lima, J. L. F. C. (2010). A thionine-based reversible redox sensor in a sequential injection system. Analytica Chimica Acta, 668, 41–46. doi:10.1016/j.aca.2010.01.060.
Ruiz-Haas, P., & Ingle, J. D., Jr. (2007). Monitoring redox conditions with flow-based and fiber-optic sensors based on redox indicators: application to reductive dehalogenation in a bioaugmented soil column. Geomicrobiology Journal, 24, 365–378. doi:10.1080/01490450701459226.
Ryu, J., Dahlgren, R. A., Gao, S., & Tanji, K. K. (2004). Characterization of redox processes in shallow groundwater of Owens Dry Lake, California. Environmental Science and Technology, 38, 5950–5957. doi:10.1021/es0306112.
Seiler, K., & Simon, W. (1992). Theoretical aspects of bulk optode membranes. Analytica Chimica Acta, 266, 73–87. doi:10.1016/0003-2670(92)85281-A.
Sotolongo, C. (2010), Fiber Optic Redox Sensors for In-Situ Environmental Sampling, M.S. thesis, Dept. of Civil & Env. Eng., Tufts University, Medford, MA.
Tratnyek, P. G., Reilkoff, T. E., Lemon, A. W., Scherer, M. M., Balko, B. A., Feik, L. M., et al. (2001). Visualizing redox chemistry: probing environmental oxidation. Reduction reactions with indicator dyes. Chemistry Educator, 6, 172–179. doi:10.1007/s00897010471a.
Van Bochove, E., Beauchemin, S., & Thériault, G. (2002). Continuous multiple measurement of soil redox potential using platinum microelectrodes. Soil Science Society American Journal, 66, 1813–1820. doi:10.2136/sssaj2002.1813.
Zhao, L., Wu, T., Lefèvre, J., Leray, I., & Delaire, J. A. (2009). Fluorimetric lead detection in a microfluidic device. Lab on a Chip, 9, 2818–2823. doi:10.1039/B904641K.
Acknowledgments
This work was supported by a Tufts University Faculty Research Award and the Tufts University Summer Scholar program. The authors also thank Professor Rob White of the Tufts University Mechanical Engineering Department for the aid in film analysis. Portions of this work were presented at the ACS Annual Meeting, ENVR Division, Boston, MA on August 22, 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chudyk, W., Sotolongo, C. & Mueller, E. A fiber-optic redox sensor for the iron(III)–iron(II) transition. Environ Monit Assess 186, 415–420 (2014). https://doi.org/10.1007/s10661-013-3386-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3386-x