Abstract
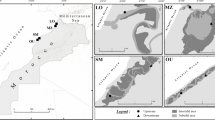
The aim of the study was to examine the spatial and temporal variations in the physicochemical parameters of seagrass meadows of the Gulf of Mannar, South India using multivariate statistical techniques, namely, cluster analysis (CA) and principal component analysis (PCA), to explore the relationship. There were clear spatial and temporal variations in physicochemical variables of the seagrass meadow of the Gulf of Mannar, but such changes were subjected to seasonal variations especially during monsoon and post-monsoon seasons. The multivariate statistical techniques, viz., CA and PCA helped in the discrimination of islands according to the physicochemical parameters of the seagrass meadows. It was inferred that electrical conductivity, nitrate, particulate organic carbon, and phosphate strongly determined the discrimination of 19 islands, respectively, upon physicochemical characteristics of their seagrass meadows. These results highlight the important role of seagrasses in the Gulf of Mannar Marine Biosphere Reserve.





Similar content being viewed by others
Abbreviations
- AT:
-
Atmospheric temperature
- SWT:
-
Surface water temperature
- MWT:
-
Meadow water temperature
- ST:
-
Sediment temperature
- SAL:
-
Salinity
- pHW:
-
Hydrogen ion concentration in water
- pHS:
-
Hydrogen ion concentration in sediment
- NO2 − :
-
Nitrite
- NO3 − :
-
Nitrate
- PO4 − :
-
Phosphate
- SiO3 − :
-
Reactive silicate
- EC:
-
Electrical conductivity
- POC:
-
Particulate organic carbon
- SND:
-
Sand
- SIL:
-
Silt
- CLY:
-
Clay
- Shi:
-
Shingle
- Kru:
-
Kurusadai
- Pul:
-
Pullivasal
- Poo:
-
Poomarichan
- Mnp:
-
Manoliputti
- Man:
-
Manoli
- Har:
-
Hare
- Mul:
-
Mulli
- Val:
-
Valai
- Thl:
-
Thalaiyari
- Apa:
-
Appa
- Vli:
-
Vaalimunai
- Ani:
-
Anaippar
- Nal:
-
Nallathanni
- Puz:
-
Puzhuvinichalli
- Kar:
-
Karachalli
- Kas:
-
Kasuwar
- Van:
-
Van
References
Adams, J. B., & Bate, G. C. (1998). The ecological implications of tolerance to salinity by Ruppia cirrhosa and Zostera capensis. Botanica Marina, 37, 449–456.
Asha, P. S., & Diwakar, K. (2007). Hydrobiology of the inshore waters off Tuticorin in the Gulf of Mannar. Journal of Marine Biological Association of India, 49, 07–11.
Bach, S. S., Borum, J., Fortes, M. D., & Duarte, C. M. (1998). Species composition and plant performance of mixed seagrass beds along a siltation gradient at Cape Bolinao, The Philippines. Marine Ecology Progress Series, 174, 247–256.
Balakrishnan Nair, N., Krishna Kumar, K., Rajasekaran Nair, J., Abdul Azis, P. K., Dhar Maraj, K., & Arunachal, M. (1983). Ecology of Indian estuaries XI. A preliminary survey of the fishery resources of the Ashtamudi estuarine system. Fishery Technology, 20, 75–83.
Bandeira, S. O. (2002). Diversity and distribution of seagrasses around Inhaca Island, southern Mozambique. South African Journal of Botany, 68, 191–198.
Sulochanan, B., & Muniyandi, K. (2005). Hydrographic parameters off Gulf of Mannar and Palk Bay during a year of abnormal rainfall. Journal of Marine Biological Association of India, 47, 198–200.
Björk, M., Uku, J., Weil, A., & Beer, S. (1999). Photosynthetic tolerances to desiccation of tropical intertidal seagrasses. Marine Ecology Progress Series, 191, 121–126.
Bulthuis, D. A. (1987). Effects of temperature on photosynthesis and growth of seagrasses. Aquatic Botany, 27, 27–40.
Burdick, D. M., Short, F. T., & Wolf, J. (1993). An index to assess and monitor the progression of wasting disease in eelgrass, Zostera marina. Marine Ecology Progress Series, 94, 83–90.
Costanza, R., et al. (1997). The value of the world’s ecosystem services and natural capital. Nature, 387, 253–260.
den Hartog, C. (1970). The seagrasses of the World. Amsterdam: North Holland. 275.
Dixon, W., & Chiswell, B. (1996). Review of aquatic monitoring program design. Water Research, 30, 1935–1948.
Duarte, C. M., Borum, J., Short, F. T., & Walker, D. I. (2005). Seagrass ecosystems: their global status and prospects. In N. V. C. Polunin (Ed.), Aquatic ecosystems: trends and global prospects. New York: Cambridge University Press.
Erftemeijer, P. L. A., & Herman, P. M. J. (1994). Seasonal changes in environmental variables, biomass, production and nutrient contents in two contrasting tropical intertidal seagrass beds in South Sulawesi, Indonesia. Oecologia, 99, 45–59.
Fletcher, S. W., & Fletcher, W. W. (1995). Factors affecting changes in seagrass distribution and diversity in the Indian River Lagoon complex between 1940 and 1992. Bulletin of Marine Science, 57, 49–58.
Fonseca, M. S., & Kenworthy, W. J. (1987). Effects of current on photosynthesis and distribution of seagrasses. Aquatic Botany, 27, 59–78.
Fortes, M. D. (1986). Taxonomy and ecology of Philippine seagrasses (p. 245). Quezon City: Ph.D. thesis, University of the Philippines, Diliman.
Ganesan, M. (1992). Ecobiology of seaweeds of the Gulf of Mannar with special reference to hydrographic and heavy metals. Ph.D. thesis, Annamalai University, pp. 164.
Ganesan, M., & Kannan, L. (1995). Seasonal distribution of intertidal seaweeds and seagrasses at two selected place of the Gulf of Mannar. Phykos, 34, 135–144.
Gopinathan, C. P., Rodrigo, J. X., Mohamed Kasim, H., & Rajagopalan, M. S. (1994). Phytoplankton pigments in relation to primary production and nutrients in the inshore waters of Tuticorin, South East coast of India. Indian Journal of Marine Science, 23, 209–212.
Govindasamy, C., Kannan, L., & Azariah, J. (2000). Seasonal variation in physico-chemical properties and primary production in the coastal water biotopes of Coramandal coast, India. Journal of Environmental Biology, 21, 1–7.
Gullstrom, M., Torre-Castro, M., Bandeira, S. O., Bjork, M., Dahlberg, M., Kautsky, N., et al. (2002). Seagrass ecosystems in the western Indian Ocean. Ambio, 31, 588–596.
Habeebrehman, H., Prabhakaran, M. P., Jacob, J., Sabu, P., Jayalakshmi, K. J., Achuthankutty, C. T., et al. (2008). Variability in biological responses influenced by upwelling events in the Eastern Arabian Sea. Journal of Marine Systems, 74, 545–560.
Hackett, H. E. (1977). Marine algae known from Maldive Islands. Atoll Research Bulletin, 210, 2–37.
Hammer, Ø., Harper, D.A.T., & Ryan, P.D. (2008). PAST—PAlaeontological STatistics, ver.1.81. http://folk.uio.no/ohammer/past.
Hemminga, M. A., & Duarte, C. M. (2000). Seagrass ecology (p. 298). United Kingdom: Cambridge University Press.
Herbert, D. A., & Fourqurean, J. W. (2009). Phosphorus availability and salinity control productivity and demography of the seagrass Thalassia testudinum in Florida Bay. Estuaries Coasts, 32, 188–201.
Hillman, K., McComb, A. J., & Walker, D. I. (1995). The distribution, biomass and primary production of the seagrass Halophila ovalis in the Swan/Canning Estuary, Western Australia. Aquatic Botany, 51, 1–54.
Houghton, J. T., Meira Filho, L. G., Callander, B. A., Harris, N., Kattenburg, A., & Maskell, K. (Eds.). (1996). Climate change 1995—the science of climate change (p. 572). New York: Cambridge University Press.
Jagtap, T. G., Komarpant, D. S., & Rodrigues, R. S. (2003). Status of a seagrass ecosystem: an ecologically sensitive wetland habitat from India. Wetlands, 23, 161–170.
Jagtap, T. G. (1991). Distribution of seagrasses along the Indian coast. Aquatic Botany, 40, 379–386.
Jagtap, T. G. (1996). Some quantitative aspects of structural components of seagrass meadows from the southeast coast of India. Botanica Marina, 39, 39–45.
Jagtap, T. G. (1998). Structure of major seagrass beds from three coral reef atolls of Lakshadweep, Arabian Sea, India. Aquatic Botany, 60, 397–408.
Jagtap, T. G., & Untawale, A. G. (1984). Chemical composition of marine macrophytes and their surrounding water and sediments from Minocoy, Lakshadweep. Indian Journal of Marine Science, 13, 123–125.
Kamermans, P., Hemminga, M. A., & de Jong, D. J. (1999). Significance of salinity and silicon levels for growth of a formerly estuarine eelgrass (Zostera marina) population (Lake Grevelingen, The Netherlands). Marine Biology, 133, 527–539.
Kannan, L., Velusamy, K., & Thangaradjou, T. (1998). Seagrasses of the Portonovo marine environment: 1. Morphological diversity in Halophila ovalis (R.Br) Hook with reference to depth & substratum. Seaweed Research & Utilization, 20, 107–113.
Kannan, R., & Kannan, L. (1996). Physicochemical characteristics of seaweed beds of the Palk Bay, southeast coast of India. Indian Journal of Marine Science, 25, 358–362.
Koch, E. W., & Dawes, C. J. (1991). Ecotypic differentiation in populations of Ruppia maritima L. germinated from seeds and cultured under algae-free laboratory conditions. Journal of Experimental Marine Biology and Ecology, 153, 145–159.
Larkum, A. W. D., Orth, R. J., & Duarte, C. M. (Eds.). (2006). Seagrasses: biology, ecology and conservation (p. 691). Dordrecht: Springer.
Lindholm, R. (1987). A practical approach to sedimentology (p. 278). London: Allen and Unwin.
Margalef, R. (1974). Ecologia. Barcelona: Ediciones Omega SA. 951.
Marichamy, R., & Siraimeetan, P. (1979). Hydrological studies in the coastal waters of Tuticorin, Gulf of Mannar. Indian Journal of Marine Science, 21, 67–76.
Masini, R. J., & Manning, C. R. (1997). The photosynthetic responses to irradiance and temperature of four meadow-forming seagrasses. Aquatic Botany, 58, 21–36.
McNeil, D., Chilvers, M., Middledorp, J., Petersons, M., & Shaw, P. (2006). Modern statistics: a graphical introduction (p. 333). Marrickville: Southwood.
McRoy, C. P., & McMillan, C. (1977). Production ecology and physiology of seagrasses. In P. C. McRoy & C. Helfferich (Eds.), Seagrass ecosystems: a scientific prospective (pp. 53–87). New York: Marcel Dekker.
Montague, C. L., & Ley, J. A. (1993). A possible effect of salinity fluctuation on abundance of benthic vegetation and associated fauna in Northeastern Florida Bay. Estuaries, 16, 703–717.
Mukai, H., Nojima, S., & Nishihira, M. (1987). Seagrass coverage and distribution in Loloata seagrass bed. In A. Hattori (Ed.), Studies on dynamics of the biological community in tropical seagrass ecosystems in Papua New Guinea: the second report (pp. 18–27). Tokyo: Ocean Research Institute, University of Tokyo.
Nyunja, J., Ntiba, M., Onyari, J., Mavuti, K., Soetaert, K., & Bouillon, S. (2009). Carbon sources supporting a diverse fish community in a tropical coastal ecosystem (Gazi Bay, Kenya). Estuarine, Coastal and Shelf Science, 83, 333–341.
Orth, R. J., & Moore, K. A. (1984). Distribution and abundance of submerged aquatic vegetation in Chesapeake Bay: an historic perspective. Estuaries, 7, 531–540.
Parsons, T. T., Matia, Y., & Lalli, C. M. (1984). A manual of chemical and biological methods for seawater analysis (p. 173). New York: Pergamon.
Petersen, C. G. J., & Boysen-Jensen, P. (1911). Valuation of the sea. I. Animal life of the sea bottom, its food and quantity. Report Dansk Biology Station, 20, 1–81.
Rajasekar, M. (2003). Physico-chemical characteristics of the Vellar estuary in relation to shrimp farming. Journal of Environmental Biology, 24, 95–101.
Rajeswari, M. (1991). Seagrass ecosystem. In R. Natarajan, S. N. Dwivedi, & S. Ramachandran (Eds.), Coastal zone management in Tamilnadu State, India (pp. 229–235). Chennai: Institute of Ocean Management, Anna University.
Rajeswari, M., & Kamala, G. (1987). Ecological observation of seagrass beds in the Gulf of Mannar, India. Environmental Conservation, 14, 265–268.
Rajeswari, M., & Kamala, G. (1988). Conservation of shallow marine communities in the Gulf of Mannar, India. Galaxea, 7, 330.
Ramamoorthy, K. (1998). Plankton characteristics of the coral reef environment of the Gulf of Mannar Biosphere Reserve (p. 143). India: Ph.D. thesis, Annamalai University.
Rasmussen, E. (1977). The wasting disease of eelgrass (Zostera marina) and its effects on environmental factors and fauna. In C. P. McRoy & C. Helfferich (Eds.), Seagrass ecosystems. A scientific perspective (pp. 1–51). New York: Marcel Dekker.
Schils, T., & Coppejans, E. (2003). Spatial variation in subtidal plant communities around the Socotra Archipelago and their biogeographic affinities within the Indian ocean. Marine Ecology Progress Series, 251, 103–114.
Short, F. T., & Neckles, H. A. (1999). The effects of global climate change on seagrasses. Aquatic Botany, 63, 169–196.
Short, F. T., & Wyllie-Echeverria, S. (1996). Natural and human-induced disturbance of seagrasses. Environmental Conservation, 23, 17–27.
Spalding, M., Taylor, M., Ravilious, C., Short, F., & Green, E. (2003). The distribution and status of seagrasses. In E. P. Green & F. T. Short (Eds.), World atlas of seagrasses (pp. 5–26). Berkeley: University of California Press.
Strickland, J. D. H., & Parsons, T. R. (1972). A practical handbook of seawater analysis (167th ed., p. 310). Canada: Bulletin of Fish Research Board.
Swinchatt, J. P. (1965). Significance of constituent, composition, texture and skeletal breakdown in some recent carbonate sediments. Sedimentary Petrology, 35, 71–90.
Tanaka, Y., & Kayanne, H. (2007). Relationship of species composition of tropical seagrass meadows to multiple physical environmental factors. Ecological Research, 22, 87–96.
Terrados, J., Duarte, C. M., Fortes, M. D., Borum, J., Agawin, N. S. R., Bach, S., et al. (1998). Changes in community structure and biomass of seagrass communities along gradients of siltation in SE Asia. Estuarine, Coastal and Shelf Science, 46, 757–768.
Thangaradjou, T. (2000). Eco-biology, culture and experimental transplantation of seagrasses of the Gulf of Mannar Biosphere. India: Reserve. Ph.D. Thesis, Annamalai University.
Thangaradjou, T., & Kannan, L. (2005). Marine sediment texture and distribution of seagrasses in the Gulf of Mannar Biosphere Reserve. Seaweed Research & Utilization, 27, 145–154.
Thangaradjou, T., & Kannan, L. (2007). Nutrient characteristics and sediment texture of the seagrass beds of the Gulf of Mannar. Journal of Environmental Biology, 28, 29–33.
Thangaradjou, T., & Kannan, L. (2008). Survival and growth of transplants of laboratory raised axenic seedlings of Enhalus acoroides (L.f.) Royle and field-collected plants of Syringodium isoetifolium (Aschers.) Dandy, Thalassia hemprichii (Ehrenb.) Aschers. and Halodule pinifolia (Miki) den Hartog. Journal of Coastal Conservation, 12, 135–143.
Uku, J., & Björk, M. (2005). Productivity aspects of three tropical seagrass species in areas of different nutrient levels in Kenya. Estuarine, Coastal and Shelf Science, 63, 407–420.
Vermaat, J. E., Agawin, N. S. R., Fortes, M. D., Uri, J. S., Duarte, C. M., Marba, N., et al. (1997). The capacity of seagrass to survive increased turbidity and siltation: the significance of growth form and light use. Ambio, 26, 499–504.
Vinithkumar, N. V., Kumeresan, S., Manjusha, M., & Balasubramanian, T. (1999). Organic matter, nutrients and major ions in the sediments of coral reefs and seagrass beds of Gulf of Mannar Biosphere Reserve, southeast coast of India. Indian Journal of Marine Science, 28, 383–393.
Walker, D. I., & Prince, K. I. T. (1987). Distribution and biogeography of seagrass species on the northwest coast of Australia. Aquatic Botany, 29, 19–32.
Wentworth, C. K. (1922). A scale of grade and class terms for clastic sediments. Journal of Geology, 30, 377–392.
Woodward, E. M. S., Rees, A. P., & Stephens, J. A. (1999). The influence of the south-west monsoon upon the nutrient biogeochemistry of Arabian Sea. Deep-Sea Research, 46, 571–591.
Zieman, J. C., Fourqurean, J. W., & Frankovich, T. A. (1999). Seagrass die-off in Florida Bay: long-term trends in abundance and growth of Turtle Grass, Thalassia testudinum. Estuaries, 22, 460–470.
Acknowledgments
The authors are grateful to Prof. T. Balasubramanian, Dean, Centre of Advanced Study in Marine Biology, Faculty of Marine Sciences and the authorities of Annamalai University for providing the necessary facilities. The authors also thank UNDP-GEF and GOMBRT for financial support and the Department of Forest, Tamil Nadu for giving permission to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arumugam, R., Kannan, R.R.R., Saravanan, K.R. et al. Hydrographic and sediment characteristics of seagrass meadows of the Gulf of Mannar Marine Biosphere Reserve, South India. Environ Monit Assess 185, 8411–8427 (2013). https://doi.org/10.1007/s10661-013-3183-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3183-6