Abstract
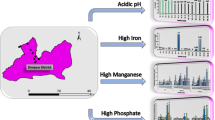
In this research, the quality of drinking well waters from 14 districts around Seoul metropolitan city, Korea was assessed by measuring a number of parameters with established guideline (e.g., arsenic, fluoride, nitrate nitrogen, benzene, 1,2-dichloroethene, dichloromethane, copper, and lead) and without such criteria (e.g., hardness, chloride ion, sulfate ion, ammonia nitrogen, aluminum, iron, manganese, and zinc). Physical parameters such as evaporation residue (or total dissolved solids) and turbidity were also measured. The importance of each parameter in well waters was examined in terms of the magnitude and exceedance frequency of guideline values established by international (and national) health agencies. The results of this study indicate that among the eight parameters with well-established guidelines (e.g., WHO), arsenic and lead (guideline value of 0.01 mg L−1 for both) recorded the highest exceedance frequency of 18 and 16 well samples ranging in 0.06–136 and 2–9 mg L−1, respectively. As such, a number of water quality parameters measured from many well waters in this urban area were in critical levels which require immediate attention for treatment and continuous monitoring.



Similar content being viewed by others
References
American Conference of Industrial Hygienists (ACGIH) (2004a). Arsenic. Threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists.
American Conference of Industrial Hygienists (ACGIH) (2004b). Benzene. Threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati, OH: American Conference of Governmental Industrial Hygienists.
Bissen, M., & Frimmel, F. H. (2003). Arsenic—a review. Part 1: occurrence, toxicity, speciation, mobility. Acta Hydrochimica et Hydrobiologica, 31, 9–18.
BNA (2001). Environmental and Safety Library on the Web. States and Territories. Washington, DC. Bureau of National Affairs Inc. http://www.esweb.bna.com/.
Drew, D. Hötzl, H. (1999) Karst hydrogeology and human activities. Impacts, consequences and implications. International Contributions to Hydrogeology (IAH). Balkema: Rotterdam, Brookfield.
EPA (1999). National recommended water quality criteria-correction. U.S. Environmental Protection Agency, Office of Water. EPA822Z99001.
EPA (2002a). National primary drinking water regulations. Washington, DC: Office of Ground Water and Drinking Water, U.S. Environmental Protection Agency. EPA816F02013. Available at: http://www.epa.gov/safewater/mcl.html.
EPA (2002b). National primary drinking water regulations. Consumer factsheet on: Copper. Wysiwyg://196/http://epa.gov/safewater/dwh/c-ioc/copper.html.
EPA (2002c). National primary drinking water regulations. Maximum contaminant level goals for inorganic contaminants. U.S. Environmental Protection Agency. Code of Federal Regulations. 40 CFR 141.51(b). Available at: http://ecfrback.access.gpo.gov.
EPA (2002d). National secondary drinking water regulations. Secondary maximum contaminant levels. U.S. Environmental Protection Agency. Code of Federal Regulations. 40 CFR 143.3. Available at: http://ecfrback.access.gpo.gov/otcg.
EPA (2003a). National primary drinking water regulations. Washington, DC: U.S. Environmental Protection Agency, Office of Ground Water and Drinking Water. EPA816F03016. Available at: http://www.epa.gov/safewater/mcl.html.
EPA (2003b). National secondary drinking water regulations. Secondary maximum contaminant levels. Washington, DC: U.S. Environmental Protection Agency. 40 CFR 143.3. Available at: http://www.epa.gov/epahome/cfr40.htm.
EPA (2004). Drinking water standards and health advisories. Washington, DC: Office of Water, U.S. Environmental Protection Agency. EPA822R04005. http://www.epa.gov/waterscience/drinking/standards/dwstandards.pdf.
EPA (2006). Drinking water standards and health advisories. Washington, DC: U.S. Environmental Protection Agency. EPA822R06013. Available at: http://www.epa.gov/waterscience/criteria/drinking/dwstandards.pdf.
FDA (2001). Beverages. Bottled water. U.S. Food and Drug Administration. Code of Federal Regulations. 21 CFR 165.110. Available at: http://www.frwebgate.access.gpo.gov/cgi.
FDA (2003). Beverages. Bottled water. Washington, DC: U.S. Food and Drug Administration. 21 CFR 165.110. Available at: http://www.access.gpo.gov/cgi-bin/cfrassemble.cgi?title=200321.
FDA (2004). Beverages. Bottled water. U.S. Environmental Protection Agency. Washington, DC: Food and Drug Administration. Code of Federal Regulations. 21 CFR 165.110. Available at: http://a257.g.akamaitech.net/7/257/2422/12feb20041500/edocket.access.gpo.gov/cfr_2004/aprqtr/pdf/21cfr165.110.pdf.
FDA (2005). Beverages. Bottled water. Final Rule. Food and Drug Administration. Code of Federal Regulations. 21 CFR 165.110. Fed Regist 70:33694-33701. Available at: http://www.fda.gov/OHRMS/DOCKETS/98fr/05-11406.pdf.
FDA (2007). Beverages. Bottled water. U.S. Food and Drug Administration. Code of Federal Regulations. 21 CFR 165.110. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm.
Gao, J. (2009). Bathymetric mapping by means of remote sensing: methods, accuracy and limitations. Progress in Physical Geography, 33(1), 103–116.
Gelinas, Y., Randall, H., Robidoux, L., & Schmit, J. P. (1996). Well water survey in two districts of Conakry (Republic of Guinea), and comparison with the piped city water. Water Research, 30, 2017–2026.
Hipsey, M. R., Brookes, J. D., Regel, R. H., Antenucci, J. P., & Burch, M. D. (2006). In situ evidence for the association of total coliforms and Escherichia coli with suspended inorganic particles in an Australian reservoir. Water, Air, and Soil Pollution, 170, 191–209.
HSDB (2003). Zinc. Environmental standards and regulations. Bethesda, MD: Hazardous Substances Data Bank. Available at: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB.htm.
HSDB (2004). Hazardous Substances Data Bank. Available at: http://toxnet.nlm.nih.gov/cgi-bin/sis/html.
IARC (2004). Overall evaluations of carcinogenicity to humans: As evaluated in IARC Monographs volumes 1–82 (at total of 900 agents, mixtures and exposures). Lyon, France: International Agency for Research on Cancer. Available at: http://www-cie.iarc.fr/monoeval/crthall.html.
IARC (2007). Agents reviewed by the IARC monographs. Volumes 1–96. (alphabetical order). Lyon, France: International Agency for Research on Cancer. Available at: http://monographs.iarc.fr/ENG/Classification/Listagentsalphorder.pdf.
IRIS (2003). Zinc and compounds. Washington, DC: Integrated Risk Information System. Available at: http://www.epa.gov/iris/.
IRIS (2004). Copper. Integrated Risk Information System. Available at: http://www.epa.gov/iris/subst/0368.htm.
IRIS (2005). Lead. U.S. Environmental Protection Agency. Washington, DC: Integrated Risk Information System. Available at: http://www.epa.gov/iris/.
IRIS (2007). Benzene. Integrated Risk Information System. Washington, DC: U.S. Environmental Protection Agency. Available at: http://www.epa.gov/iris/subst/index.html.
IRIS (2008). Manganese. Integrated Risk Information System. Washington, DC: U.S. Environmental Protection Agency. Available at: http://www.epa.gov/iris/subst/index.html.
Kaown, D., Koh, D. C., Mayer, B., & Lee, K.-K. (2009a). Identification of nitrate and sulfate sources in groundwater using dual stable isotope approaches for an agricultural area with different land use (Chuncheon, mid-eastern Korea). Agriculture, Ecosystems & Environment, 132, 223–231.
Kaown, D., Koh, D.-C., Mayer, B., & Lee, K.-K. (2009b). Identification of nitrate and sulfate sources in groundwater using dual stable isotope approaches for an agricultural area with different land use (Chuncheon, mid-eastern Korea). Agriculture, Ecosystems & Environment, 132, 223–231.
Mahler, B. J., Personné, J.-C., Lods, G. F., & Drogue, C. (2000). Transport of free and particulate-associated bacteria in karst. Journal of Hydrology, 238, 179–193.
Ng, A., & Patterson, C. (1981). Natural concentrations of lead in ancient Arctic and Antarctic ice. Geochimica et Cosmochimica Acta, 45, 2109–2121.
NTP (2005). Report on carcinogens. 11th ed. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program. Available at: http://ntpserver.niehs.nih.gov/ntp/roc/toc11.html
Pritchard, M., Mkandawire, T., & O’Neill, J. G. (2007). Biological, chemical and physical drinking water quality from shallow wells in Malawi: case study of Blantyre, Chiradzulu and Mulanje. Physics and Chemistry of the Earth, 32, 1167–1177.
Pronk, M., Goldscheider, N., & Zopfi, J. (2007). Particle-size distribution as indicator for fecal bacteria contamination of drinking water from karst springs. Environmental Science & Technology, 41(24), 8400–8405.
Reimann, C., & Banks, D. (2004). Setting action levels for drinking water: are we protecting our health or our economy (or our backs!)? Science of the Total Environment, 332, 13–21.
Tebutt, T.H.Y. (1998). Principles of water quality control, fifth edn. Oxford: Butterworth–Heinemann, ISBN 0 7506 3658 0.
WHO (2001). Guidelines for drinking-water quality. Fluoride. World Health Organization. Available at: http://www.who.int/water_sanitation_lth/GDWQ/Chemicals/fluoridefull/html.
WHO. (2003a). Copper in drinking-water (Background document for preparation of WHO Guidelines for drinking-water quality). Geneva: World Health Organization. WHO/SDE/WSH/03.04/88.
WHO. (2003b). 1,2-dichloroethene in drinking-water (Background document for preparation of WHO Guidelines for drinking-water quality). Geneva: World Health Organization. WHO/SDE/WSH/03.04/72.
WHO. (2003c). Dichloromethane in drinking-water (Background document for preparation of WHO Guidelines for drinking-water quality). Geneva: World Health Organization. WHO/SDE/WSH/03.04/18.
WHO. (2003d). Chloride in drinking-water (Background document for preparation of WHO Guidelines for drinking-water quality). Geneva: World Health Organization. WHO/SDE/WSH/03.04/3.
WHO. (2003e). Iron in drinking-water (Background document for preparation of WHO Guidelines for drinking-water quality). Geneva: World Health Organization. WHO/SDE/WSH/03.04/08.
WHO. (2003f). Sulfate in drinking-water (Background document for preparation of WHO Guidelines for drinking-water quality). Geneva: World Health Organization. WHO/SDE/WSH/03.04/114.
WHO. (2003g). Ammonia in drinking-water (Background document for preparation of WHO Guidelines for drinking-water quality). Geneva: World Health Organization. WHO/SDE/WSH/03.04/1.
WHO. (2003h). Total dissolved solids in drinking-water (Background document for preparation of WHO Guidelines for drinking-water quality). Geneva: World Health Organization. WHO/SDE/WSH/03.04/16.
WHO (2004). Guidelines for drinking-water quality. 3rd edn. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/water_sanitation_health/dwq/gdwq3/en/.
WHO. (2010). Aluminium in drinking-water (Background document for preparation of WHO Guidelines for drinking-water quality). Geneva: World Health Organization. WHO/SDE/WSH/03.04/53.
WHO (2011a). Guidelines for drinking water quality. 4th edn. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/
WHO. (2011b). Nitrate and nitrite in drinking-water (Background document for preparation of WHO Guidelines for drinking-water quality). Geneva: World Health Organization. WHO/HSE/AMR/07.01/16/Rev/1.
WHO. (2011c). Hardness in drinking-water (Background document for preparation of WHO Guidelines for drinking-water quality). Geneva: World Health Organization. WHO/HSE/WSH/10.01/10/Rev/1.
Acknowledgment
This study was supported by a National Research Foundation of Korea grant funded by the Ministry of Education, Science and Technology (No. 2010-0007876).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Kim, KH., Susaya, J.P., Park, C.G. et al. Comprehensive monitoring of drinking well water quality in Seoul metropolitan city, Korea. Environ Monit Assess 185, 6353–6378 (2013). https://doi.org/10.1007/s10661-012-3030-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-3030-1