Abstract
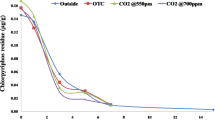
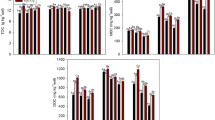
An experiment was conducted in open-top chambers (OTC) to study the effect of elevated CO2 (580 ± 20 μmol mol−1) on azoxystrobin degradation and soil microbial activities. Results indicated that elevated CO2 did not have any significant effect on the persistence of azoxystrobin in rice-planted soil. The half-life values for the azoxystrobin in rice soils were 20.3 days in control (rice grown at ambient CO2 outdoors), 19.3 days in rice grown under ambient CO2 atmosphere in OTC, and 17.5 days in rice grown under elevated CO2 atmosphere in OTC. Azoxystrobin acid was recovered as the only metabolite of azoxystrobin, but it did not accumulate in the soil/water and was further metabolized. Elevated CO2 enhanced soil microbial biomass (MBC) and alkaline phosphatase activity of soil. Compared with rice grown at ambient CO2 (both outdoors and in OTC), the soil MBC at elevated CO2 increased by twofold. Elevated CO2 did not affect dehydrogenase, fluorescein diacetate, and acid phosphatase activity. Azoxystrobin application to soils, both ambient and elevated CO2, inhibited alkaline phosphates activity, while no effect was observed on other enzymes. Slight increase (1.8–2 °C) in temperature inside OTC did not affect microbial parameters, as similar activities were recorded in rice grown outdoors and in OTC at ambient CO2. Higher MBC in soil at elevated CO2 could be attributed to increased carbon availability in the rhizosphere via plant metabolism and root secretion; however, it did not significantly increase azoxystrobin degradation, suggesting that pesticide degradation was not the result of soil MBC alone. Study suggested that increased CO2 levels following global warming might not adversely affect azoxystrobin degradation. However, global warming is a continuous and cumulative process, therefore, long-term studies are necessary to get more realistic assessment of global warming on fate of pesticide.



Similar content being viewed by others
References
Adetutu, E. M., Ball, A. S., & Osborn, A. M. (2008). Azoxystrobin and soil interactions: Degradation and impact on soil bacterial and fungal communities. Journal of Applied Microbiology, 105, 1777–1790.
Bailey, S. W. (2003). Climate change and decreasing herbicide persistence. Pest Management Science, 60, 158–162.
Bazot, S., Ulff, L., Blum, H., Nguyen, C., & Robin, C. (2006). Effects of elevated CO2 concentration on rhizodeposition from Lolium perrene grown on soil exposed to 9 years of CO2 enrichment. Soil Biology and Biochemistry, 38, 729–736.
Bending, G. D., Lincoln, S. D., & Edmondson, R. N. (2006). Spatial variation in the degradation rate of the pesticides isoproturon, azoxystrobin and diflufenican in soil and its relationship with chemical and microbial properties. Environmental Pollution, 139, 279–287.
Bending, G. D., Rodriguez-Cruz, M. S., & Lincoln, S. D. (2007). Fungicide impacts on microbial communities in soil with contrasting management histories. Chemosphere, 69, 82–88.
Black, C.A. (1965). Methods of soil analysis (part- 1 and 2) American Soc. Agron. Madison, Wisconsin, U.S.A.
Bloomfield, J. P., Williams, R. J., Gooddy, D. C., Cape, J. N., & Guha, P. (2006). Impact of climate change on the fate and behavior of pesticides in surface and ground water—A UK perspective. Science of the Total Environment, 369, 163–177.
Boxall, A. B. A., Hardy, A., Beulke, S., Boucard, T., Burgin, L., Falloon, P. D., et al. (2009). Climate change's effect on indirect exposure to agricultural pathogens/chemicals: Impacts of climate change on transport, fate, and exposure. Environmental Health Perspective, 117, 508–514.
Carney, K. M., Hungate, B. A., Drake, B. G., & Megonigal, J. P. (2007). Altered soil microbial community at elevated CO2 leads to loss of soil carbon. Proceedings of National Academy of Sciences USA, 104, 4990–4995.
Casida, L. E., Klein, D. A., & Santoro, T. (1964). Soil dehydrogenase activity. Soil Science, 98, 371–376.
Das, S., Bhattacharyya, P., & Adhya, T. K. (2011). Interaction effects of elevated CO2 and temperature on microbial biomass and enzyme activities in tropical rice soils. Environment Monitoring and Assessment, 182, 555–569.
De Graaff, M., Van Groenigen, K., & Six, J. (2006). Interactions between plant growth and soil nutrient cycling under elevated CO2: A meta analysis. Global Change Biology, 12, 2077–2091.
Drigo, B., Kowalchuk, G. A., & van Veen, J. A. (2008). Climate change goes underground: Effects of elevated atmospheric CO2 on microbial community structure and activities in the rhizosphere. Biology and Fertility of Soils, 44, 667–679.
Gajbhiye, V. T., Gupta, S., Mukherjee, I., Singh, S. B., Singh, N., Dureja, P., et al. (2011). Persistence of azoxystrobin in/on grapes and soil in different grapes growing areas of India. Bulletin of Environmental Contamination and Toxicology, 86, 90–94.
Ghosh, R. K., & Singh, N. (2009). Leaching behaviour of azoxystrobin in soil columns. Pest Management Science, 65, 1009–1014.
Green, V. S., Stott, D. E., & Diaek, M. (2006). Assay of fluorescene diacatae hydrolytic activity optimization of soil samples. Soil Biology and Biochemistry, 38, 693–701.
Henry, H. A. L., Juarez, J. D., Field, C. B., & Vitousek, P. M. (2005). Interactive effects of elevated CO2, N deposition and climate change on extracellular enzyme activity and soil density fractionation in a California annual grassland. Global Change Biology, 11, 1–8.
Hill, P. W., Marshall, C., Williams, G. G., Blum, H., Harmens, H., Jones, D. L., et al. (2007). The fate of photosynthetically fixed carbon in Lolium perenne grassland as modified by elevated CO2 and sward management. New Phytology, 173, 766–777.
Hoque, M. M., Inubushi, K., Miura, S., Kobayashi, K., Kim, H. Y., & Okada, M. (2001). Biological dinitrogen fixation and soil microbial biomass carbon as influenced by free-air carbon dioxide enrichment (FACE) at three levels of nitrogen fertilization in a paddy field. Biology and Fertility of Soils, 34, 453–459.
Inubushi, K., Hoque, M., Miura, S., Kobayashi, K., Kim, H. Y., & Okada, M. (2001). Effect of free-air CO2 enrichment (FACE) on microbial biomass in paddy field soil. Soil Science and Plant Nutrition, 47, 737–745.
Inubushi, K., Mizuno, T., Lou, Y., Hasegawa, T., Lin, Y., Cheng, W., Kobayashi, K., Okada, M. (2010). Microbial biomass and activities in a Japanese paddy soil with differences in atmospheric CO2 enrichment, soil/water warming and rice cultivars. 19th World Congress of Soil Science, Soil Solutions for a Changing World, 1–6 August 2010, Brisbane, Australia.
Inubushi, K., Cheng, W., Mizuno, T., Lou, Y., Hasegawa, T., Sakai, H., et al. (2011). Microbial biomass carbon and methane oxidation influenced by rice cultivars and elevated CO2 in a Japanese paddy soil. European Journal of Soil Science, 62, 69–73.
IPCC. (2007). Climate Change 2007: The physical science basis, contribution of working group I to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press.
Jackson, M. L. (1967). Soil chemical analysis. New Delhi: Prentice Hall Inc.
Janus, L., Angeloni, N., McCormack, J., Rier, S., Tuchman, N., & Kelly, J. (2005). Elevated atmospheric CO2 alters soil microbial communities associated with trembling aspen (Populus tremuloides) roots. Microbial Ecology, 50, 102–109.
Joseph, R.S.I. (2000) Metabolism and degradation of the fungicide azoxystrobin. In: Book of Abstracts of 219th ACS National Meeting, ACS: American Clinical Society National Meeting, San Francisco, CA, March 26–30, 2000, Washington DC.
Kang, H., Kim, S. W., Fenner, N., & Freeman, C. (2005). Shifts of soil enzyme activities in wetlands exposed to elevated CO2. Science of the Total Environment, 337, 207–212.
Krull, E. S., Skjemstad, J. O., Burrows, W. H., Bray, S. G., Wynn, J. G., Bol, R., et al. (2005). Recent vegetation changes in central Queensland, Australia: Evidence from δ13C and 14 C analyses of soil organic matter. Geoderma, 126, 241–259.
Lesaulnier, C., Papamichail, D., McCorkle, S., Ollivier, B., Skiena, S., Taghavi, S., et al. (2008). Elevated atmospheric affects soil microbial diversity associated with trembling aspen. Environmental Microbiology, 10, 926–941.
Li, X., Han, S., Guo, Z., Shao, D. & Xin, L. (2010) Changes in soil microbial biomass carbon and enzyme activities under elevated CO2 affect fine root decomposition processes in a Mongolian oak ecosystem. Soil Biology and Biochemistry, 42, 1101-1107.
Li, Z., Yagi, K., Sakai, H., & Kobayashi, K. (2004). Influence of elevated CO2 and nitrogen nutrition on rice plant growth, soil microbial biomass, dissolved organic carbon and dissolved CH4. Plant and Soil, 258, 81–90.
McCarthy, J.J., Canziani, O.F., Leary, N.A., Dokken, D.J., White, K.S. (2001). Climate change: Impacts adaptation and vulnerability. Third Assessment Report of Intergovernmental Panel for Climate Change, Cambridge University Press, Cambridge.
Pal, M., Karthikeyapandian, V., Jain, V., Srivastava, A. C., Raj, A., & Sengupta, U. K. (2004). Biomass production and nutritional levels of berseem (Trifolium alexandrium) grown under elevated CO2. Agriculture Ecosystem and Environment, 101, 31–38.
Singh, N., & Singh, S. B. (2010). Effect of moisture and compost on fate of azoxystrobin in soils. Journal of Environment Science and Health, B45, 676–681.
Tabatabai, M. A., & Bremner, J. M. (1969). Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biology and Biochemistry, 1, 301–307.
Vance, E. D., Broke, P. C., & Jenkinson, D. S. (1987). Microbial biomass measurements in forest soils: The use of chloroform fumigation–incubation method in strongly acidic soils. Soil Biology and Biochemistry, 19, 697–702.
Williams, J. R., Richardson, C. W., & Griggs, R. H. (1992). The weather factor: Incorporating weather variance into computer simulation. Weed Technology, 6, 731–735.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manna, S., Singh, N. & Singh, V.P. Effect of elevated CO2 on degradation of azoxystrobin and soil microbial activity in rice soil. Environ Monit Assess 185, 2951–2960 (2013). https://doi.org/10.1007/s10661-012-2763-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2763-1