Abstract
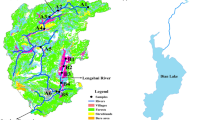
Phosphorus (P) sorption by sediments may play a vital role in buffering P concentration in the overlying water column. To characterize P sorption–desorption in the river bed sediments, 17 bed sediment samples collected from Abshineh river, in a semi arid region, Hamedan, western Iran were studied through a batch experiment and related to sediment composition. The sorbed fraction ranged from 4.4% to 5.4% and from 38.5% to 86.0% of sorption maxima when 20 and 1,500 mg P kg−1, respectively, was added to the sediment samples. Phosphorus sorption curves were well fitted to the Langmuir model. Zero equilibrium P concentration ranged from 0.10 to 0.51 mg P l−1 and varied with sediment characteristics. Phosphorus desorption differed strongly among the studied bed sediments and ranged from 10.8% to 80.2% when 1,500 mg P kg−1 was added. The results of the geochemical modelling indicated that even under low P addition (2 mg l−1), the solutions are mainly saturated with respect to hydroxyapatite and ß-tricalcium phosphate minerals and undersaturated with respect to other Ca and Mg minerals, whereas under higher P addition (150 mg l−1), most Ca–P solid phases, except the most soluble mineral (brushite), will likely precipitate. A Langmuir sorption maximum was positively correlated with carbonate calcium. Estimated P retention capacity of the bed sediments are generally lower and zero equilibrium P concentration values higher in upstream sites than at the downstream sites, suggesting that sediments in upstream and downstream may act as source and sink of P, respectively.




Similar content being viewed by others
References
Allison, J. D., Brown, D. S., & Novo-Gradac, K. J. (1991). MINTEQA2/PRODEFA2, a geochemical assessment model for environmental systems: Version 3.0 users manual. EPA/600/3-91/021. Athens: US Environmental Protection Agency.
Amer, F., Mahmoud, A. A., & Sabet, V. (1985). Zeta potential and surface area of calcium carbonate as related to phosphate sorption. Soil Science Society Of America Journal, 49, 1137–1142.
An, W. C., & Li, X. M. (2009). Phosphate adsorption characteristics at the sediment-water interface and phosphorus fractions in Nansi lake, China. Environmental Monitoring and Assessment, 148, 173–184.
Axt, J. R., & Walbridge, M. R. (1999). Phosphate removal capacity of palustrine forested wetlands and adjacent uplands in Virginia. Soil Science Society of America Journal, 63, 1019–1031.
Baharifar, A., Moinevaziri, H., Bellon, H., & Pique, A. (2004). The crystalline complexes of Hamadan (Sanandaj-Sirjan zone, western Iran): Metasedimentary Mezoic sequences affected by Late Cretaceous tectono-metamorphic and plutonic events. Comptes Rendus Geoscience, 336, 1443–1452.
Barrow, N. J. (1980). Evaluation and utilization of residual phosphorus in soils. In F. E. Khasawneh, E. C. Sample, & E. J. Kamprath (Eds.), The role of phosphorus in agriculture (pp. 330–360). Madison: American Society of Agronomy.
Barrow, N. J. (1989). Modelling the effects of pH on phosphate sorption by soils. Journal of Soil Science, 35, 283–297.
Berg, U., Neumann, T., Donnert, D., Nüesch, R., & Stüben, D. (2004). Sediment capping in eutrophic lakes—efficiency of undisturbed calcite barriers to immobilize phosphorus. Applied Geochemistry, 19, 1759–1771.
Bolster, C. H. (2008). Revisiting a statistical shortcoming when fitting the Langmuir model to sorption data. Journal of Environmental Quality, 3, 1986–1992.
Bolster, C. H., & Hornberger, G. M. (2007). On the use of linearized Langmuir equations. Soil Science Society of America Journal, 71, 1796–1806.
Borggaard, O. K., Jorgensen, S. S., Moberg, J. P., & Raben-Lange, B. (1990). Influence of organic matter on phosphate adsorption by aluminium and iron oxides in sandy soils. European Journal of Soil Science, 41, 443–449.
Borggaard, O. K., Raben-Lange, B., Gimsing, A. L., & Strobel, B. W. (2004). Influence of humic substances on phosphate adsorption by aluminium and iron oxides. Geoderma, 127, 270–279.
Bridgham, S. D., Updegraff, K., & Pastor, J. (2001). A comparison of nutrient availability indices along an ombrotrophic-minerotrophic gradient in Minnesota wetlands. Soil Science Society of America Journal, 65, 259–269.
Bubba, D. M., Arias, C. A., & Brix, H. (2003). Phosphorus adsorption maximum of sands for use as media in subsurface flow constructed reed beds as measured by the Langmuir isotherm. Water Research, 37, 3390–3400.
Camargo, J. A., Alonso, A., & de la Puente, M. (2005). Eutrophication downstream from small reservoirs in Mountain Rivers of Central Spain. Water Research, 39, 3376–3384.
Caraco, N. F., Cole, J. J., & Likens, G. E. (1989). Evidence of sulphate-controlled phosphorus release from sediments of aquatic systems. Nature, 341, 316–318.
Carreira, J. A., Viñegla, B., & Lajtha, K. (2006). Secondary CaCO3 and precipitation of P–Ca compounds control the retention of soil P in arid ecosystems. Journal of Arid Environments, 64, 460–473.
Castro, B., & Torrent, J. (1998). Phosphate sorption by calcareous Vertisols and Inceptisols as evaluated from extended P-sorption curves. European Journal of Soil Science, 49, 661–667.
Freeman, J. S., & Rowell, D. L. (1981). The adsorption and precipitation of phosphate onto calcite. Journal of Soil Science, 32, 75–84.
Froelich, P. N. (1988). Kinetic control of dissolved phosphate in natural rivers and estuaries: A primer on the phosphate buffer mechanism. Limnology and Oceanography, 33, 649–668.
Gustafsson, J.P. (2005). Visual MINTEQ ver 2.32. Stokholm, Sweden: Department of Land and Water Resources Engineering, Royal Institute of Technology. http://hem.bredband.net/b108693.
Haggard, B. E., Stanley, E. H., & Hyler, R. (1999). Sediment-phosphorus relationships in three Northcentral Oklahoma streams. Transaction of ASAE (American Society of Agricultural Engineers), 42, 1709–1714.
Heathwaite, A. L., & Johnes, P. J. (1996). Contribution of nitrogen species and phosphorus fractions to stream water quality in agricultural catchments. Hydrological Processes, 10, 971–983.
Holford, I. C. R. (1979). Evaluation of soil phosphate buffering indices. Australian Journal of Soil Research, 17, 495–504.
House, W. A., & Denison, F. H. (2000). Factors influencing the measurement of equilibrium phosphate concentrations in river sediments. Water Research, 34, 1187–1200.
Jalali, M. (2007a). Phosphorous status and sorption characteristics of some calcareous soils of Hamadan, western Iran. Environmental Geology, 53, 365–374.
Jalali, M. (2007b). Hydrochemical identification of groundwater resources and their changes under the impacts of human activity in the Chah basin in western Iran. Environmental Monitoring and Assessment, 130, 347–364.
Jalali, M. (2009). Phosphorous concentration, solubility and species in the groundwater in a semi-arid basin, southern Malayer, western Iran. Environmental Geology, 57, 1011–1020.
Jalali, M. (2010). Phosphorus fractionation in river sediments, Hamadan, western Iran. Soil and Sediment Contamination, 19, 560–572.
Jarvie, H. P., Jürgens, M. D., Williams, R. J., Neal, C., Davies, J. J. L., Barrett, C. (2005). Role of river bed sediments as sources and sinks of phosphorus across two major eutrophic UK river basins: The Hampshire Avon and Herefordshire Wye. Journal of Hydrologic Engineering, 304, 51–74.
Kim, L. H., Choi, E., & Stenstrom, M. K. (2003). Sediment characteristics, phosphorus types and phosphorus release rates between river and lake sediments. Chemosphere, 50, 53–61.
Kitano, Y., Okumura, M., & Idogaki, M. (1978). Uptake of phospahate ions by calcium carbonate. Geochemical Journal, 12, 29–37.
Lai, M. Y. F., & Lam, K. Ch. (2008). Phosphorus retention and release by sediments in the eutophic Mai Po Marshes, Hong Kong. Marine Pollution Bulletin, 57, 349–356.
Lair, G. J., Zehetner, Z., Khan, Z. H., & Gerzabek, M. H. (2009). Phosphorus sorption–desorption in alluvial soils of a young weathering sequence at the Danube River. Geoderma, 149, 39–44.
Lake, B. A., Coolidge, K. M., Norton, S. A., & Amirbahman, A. (2007). Factors contributing to the internal loading of phosphorus from anoxic sediments in six Maine, USA, lakes. Science of the Total Environment, 373, 534–541.
Li, B. G., & Guo, B. S. (2006). Chemical forms of inorganic phosphorus in sediments in the middle of the Yellow river. Journal of Agro-Environmental Science, 25, 1607–1610.
Lindsay, W. L. (1979). Chemical equilibria in soils. New York: Wiley.
Lindsay, W. L., Vlek, P. L. G., & Chien, S. H. (1989). Phosphate minerals. In J. B. Dixon & S. B. Weed (Eds.), Minerals in soil environments (pp. 1089–1130). Madison: Soil Science Society of America.
Litaor, M. I., Reichmann, O., Haim, A., Auerswald, K., & Shenker, M. (2005). Sorption characteristics of phosphorus in peat soils of a semiarid altered wetland. Soil Science Society of America Journal, 69, 1658–1665.
Loeppert, R. H., & Inskeep, W. P. (1996). Iron. In D. L. Sparks (Ed.), Methods of soil analysis: Chemical methods, Part 3 (pp. 639–664). Madison: ASA and SSSA.
Lopez, P., Lluch, X., Vidal, M., & Morgui, J. A. (1996). Adsorption of phosphorus on sediments of the Balearic Islands (Spain) related to their composition. Estuarine, Coastal and Shelf Science, 42, 185–196.
Lottig, N. R., & Stanley, E. H. (2007). Benthic sediment influence on dissolved phosphorus concentrations in a headwater stream. Biogeochemical, 84, 297–309.
Machesky, M. L., Holm, T. R., & Slowikowski, J. A. (2010). Phosphorus speciation in stream bed sediments from an agricultural watershed: Solid-phase associations and sorption behavior. Aquatic Geochemistry, 16, 639–662.
Makris, K. C., Harris, W. G., O’Connor, J. A., & EI-Shall, H. (2005). Long-term phosphorus effects on evolving physicochemical properties of iron and aluminum hydroxides. Journal of Colloid and Interface Science, 287, 552–560.
Matar, A., Torrent, J., & Ryan, J. (1992). Soil and fertilizer phosphorus and crop responses in the dryland Mediterranean zone. Advance in Soil Science, 18, 81–146.
McDowell, R., Sharpley, A., Brookes, P. H., & Poulton, P. (2001). Relationship between soil test phosphorus and phosphorus release to solution. Soil Science, 166, 137–149.
Mehra, O. P., & Jackson, M. L. (1960). Iron oxide removal from soils and clays by dithionite-citrate systems buffered with sodium bicarbonate. Clay and Clay Mineral, 7, 317–327.
Murphey, J., & Riley, J. P. (1962). A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta, 27, 31–36.
Nwoke, O. C., Vanlauwe, B., Diels, J., Sanginga, N., Osonubi, O., & Merckx, R. (2003). Assessment of labile phosphorus fractions and adsorption characteristics in relation to soil properties of West African savanna soils. Agriculture, Ecosystems & Environment, 100, 285–294.
Olila, O. G., & Redy, K. R. (1997). Influence of redox potential on phosphorus uptake by sediments in two sub-tropical eutrophic lakes. Hydrobiologia, 345, 45–57.
Olsen, S. L., & Sommers, L. E. (1982). Phosphorus. In A. L. Page et al. (Eds.), Methods of soil analysis, Part 2 (pp. 403–427). Madison: American Society of Agronomy.
Pansu, M., & Gautheyrou, J. (2006). Handbook of soil analysis, mineralogical, organic and inorganic analysis. Heidelberg: Springer-Verlag Berli.
Peng, J.-F., Wang, B.-Z., Song, Y.-H., Yuan, P., & Liu, Z. (2007). Adsorption and release of phosphorus in the surface sediment of a wastewater stabilization pond. Ecological Engineering, 31, 92–97.
Perry, J. J., Staley, J. T., & Lory, S. (2002). Microbial life. Sunderland: Sinauer Associates. 811.
Raven, K. P., & Hossner, L. R. (1994). Sorption and desorption quantity-intensity parameters related to plant-available soil phosphorus. Soil Science Society of America Journal, 58, 405–410.
Richardson, C. J., & Vaithiyanathan, P. (1995). Phosphorus characteristics of Everglade soils along a eutrophic gradient. Soil Science Society of America Journal, 59, 1782–1788.
Rowell, D. L. (1994). Soil science: Methods and applications. Harlow: Longman Scientific and Technical.
Sanyal, S. K., & De Datta, S. K. (1991). Chemistry of phosphorus transformations in soil. Advances in Soil Sciences, 16, 1–20.
Sepahi, A. (1999). Petrology of the Alvand plutonic complex with special reference on granitoids. Ph.D. Thesis. Tehran, Iran: Tarbiat-Moallem University. (in Persian).
Sharpley, A. N. (1996). Availability of residual phosphorus in manured soils. Soil Science Society of America Journal, 60, 1459–1466.
Sharpley, A. N., & Smith, S. J. (1989). Prediction of soluble phosphorus transport in agricultural runoff. Journal of Environmental Quality, 18, 313–316.
Sims, J. T., Simard, R. R., & Joern, B. C. (1998). Phosphorus losses in agricultural drainage: Historical perspective and current research. Journal of Environmental Quality, 27, 277–293.
Smith, D. R., Haggard, B. E., Warnemuende, E. A., & Huang, C. (2005). Sediment phosphorus dynamics for three tile fed drainage ditches in Northeast Indiana. Agricultural Water Management, 71, 19–32.
Stutter, M. I., & Lumsdona, D. J. (2008). Interactions of land use and dynamic river conditions on sorption equilibria between benthic sediments and river soluble reactive phosphorus concentrations. Water Research, 42, 4249–4260.
Sui, Y., Thompson, M. L., & Mize, C. W. (1999). Redistribution of biosolids-derived total P applied to a Mollisol. Journal of Environmental Quality, 28, 1068–1074.
Tian, J.-R., & Zhou, P.-J. (2008). Phosphorus fractions and adsorption characteristics of floodplain sediments in the lower reaches of the Hanjiang river, China. Environmental Monitoring and Assessment, 137, 233–241.
Tiessen, H., & Moir, J. O. (1993). Characterization of available P by sequential extraction. In M. R. Carter (Ed.), Soil sampling and methods of analysis (pp. 75–86). Boca Raton: Canadian Society of Soil Science (Lewis Publishers).
Torrent, J. (1997). Interactions between phosphate and iron oxide. In K. Auerswald, H. Stanjek, & J. M. Bigham (Eds.), Soils and environment, advance geology ecology (Vol. 30, pp. 321–344). Reiskirchen: Catena Verlag.
Tu, C., Zheng, C. R., & Chen, K. M. (2002). Effect of heavy metals on phosphorus retention by typic udic ferrisols: Equilibrium and kinetics. Pedosphere, 12, 15–24.
van Riemsdjik, W. H., Weststrate, F. A., & Bolt, G. H. (1975). Evidence of a new aluminium phosphate phase from reaction rate of phosphate with aluminium hydroxide. Nature, 257, 473–474.
Varinderpal-Singh, Dhillon, N. S., & Brar, B. S. (2006). Influence of long-term use of fertilizers and farmyard manure on the adsorption-desorption behaviour and bioavailability of phosphorus in soils. Nutrient Cycling in Agroecosystems, 75, 67–78.
Villapando, R. R., & Graetz, D. A. (2001). Phosphorus sorption and desorption properties of the spodic horizon from selected Florida spodosols. Soil Science Society of America Journal, 65, 331–339.
Wang, S. R., Jin, X. C., Bu, Q. Y., Zhou, X. N., & Wu, F. C. (2006). Effects of particle size, organic matter and ionic strength on the phosphate sorption in different tropic lake sediments. Journal of Hazardous Materials, 128, 95–105.
Wang, S. R., Jin, X. C., Zhao, H. C., Zhou, X. N., & Wu, F. C. (2007). Effect of organic matter on the sorption of dissolved organic and inorganic phosphorus in lake sediments. Colloids and Surfaces A, 297, 154–162.
Wang, Y., Shen, Z., Niu, J., & Liu, R. (2009). Adsorption of phosphorus on sediments from the Three-Gorges Reservoir (China) and the relation with sediment composition. Journal of Hazardous Materials, 162, 92–98.
Yang, Y. G., He, Z. L., Lin, Y., & Stoffella, P. J. (2010). Phosphorus availability in sediments from a tidal river receiving runoff water from agricultural fields. Agricultural Water Management, 97, 1722–1730.
Zhuan-xi, L., Bo, Z., Jia-liang, T., & Tao, W. (2009). Phosphorus retention capacity of agricultural headwater ditch sediments under alkaline condition in purple soils area, China. Ecological Engineering, 35, 57–64.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jalali, M., Peikam, E.N. Phosphorus sorption–desorption behaviour of river bed sediments in the Abshineh river, Hamedan, Iran, related to their composition. Environ Monit Assess 185, 537–552 (2013). https://doi.org/10.1007/s10661-012-2573-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2573-5