Abstract
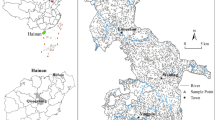
Speciation determines toxicity, transport pathways and residence time of a metal in different compartments of the environment. This study investigated the speciation of mercury in soils, derived from sites known for dumping of mine wastes in the Bibiani–Anwiaso–Bekwai district, a gold mining community of the Western Region of Ghana. Soil samples were taken from the surface; depths of 20, 40 and 60 cm from mine waste at both abandoned and active mine sites. Each sample was analysed for total mercury, organic mercury and elemental mercury. After sample treatment, digestion and reduction with stannous chloride (SnCl2), total mercury content was determined using the Inductively Coupled Plasma—Optical Emissions Spectrometer (ICP–OES). Organic mercury content was determined employing a differential technique after disposing of elemental mercury by heating. Total mercury content in samples ranged from 0.067 to 0.876 mg/kg for surface soils. The same soil of depths 20, 40 and 60 cm had total mercury from 0.102 to 1.066, 0.037 to 4.037 and 0.191 to 4.998 mg/kg, respectively. For organic mercury, concentrations range from 0.012 to 0.260 mg/kg for surface soil. Soil depths of 20, 40 and 60 cm had organic mercury concentrations from 0.016 to 0.653, 0.041 to 1.093 and 0.101 to 2.546 mg/kg respectively. Elemental mercury concentrations in surface soils, soils at depths of 20, 40 and 60 cm ranged from 0.043 to 0.780; 0.017 to 0.749; 0.014 to 2.944 and 0.009 to 2.452 mg/kg respectively. Among the sites studied, only galamsey tailings (GM) showed a trend of increasing total mercury level with increasing depth. For the other sites, trends were not defined. There has been no defined trend for elemental mercury with depth at any of the sampling sites. Just as with total mercury, it was only GM that showed an increasing trend of organic mercury concentration with depth.















Similar content being viewed by others
References
Bertsch, P. M., & Bloom, P. R. (1996). Aluminum. In D. L. Sparks (Ed.), Methods of soil analysis, part 3. Chemical methods-ASA and SSSA Book Series no. 5, Chp. 18, (pp. 517–549). Madison, WI.
Biester, H. (1994). Application of a temperature controlled pyrolysis method for the detection of Hg-species in soils and sediments (Moglichkeiten der Anwendungeines temperaturgesteuerten Pyrolyseverfahrens zur Bestimmung der Bindungsform des Quecksilbers in Boden und Sedimenten). Heidelberger Geowissenschaftliche Abhandlungen Band, Heidelberg, 75, 156.
Burgess, N. M., & Braune, B. M. (2001). Increasing trends in mercury concentrations in Atlantic and Arctic seabird eggs in Canada In W. Baeyens, R. Ebinghaus, & O. Valiliev (Eds.), Global and regional mercury cycles: Sources, fluxes and mass balances. NATO ASI Series, 2. Environment - Vol. 21.
Carpi, A. (1997). The surface/atmosphere exchange of elemental and methyl mercury over background and municipal sewage sludge amended soil. Ithaca, New York: Cornell University.
Fernández-Martínez, R., Loredo, J., Ordóñez, A., & Rucandio, M. I. (2005). Physicochemical characterization and mercury speciation of particle-size soil fractions from an abandoned mining area in Mieres, Asturias (Spain). Environmental Pollution 142(2)July 2006:217–226.
Grubaugh, K. (2002). Profile of Ghana's mining industry. Mining Engineering, 55(1).
Hossner, L. R. (1996). Dissociation for total elemental analysis. In D. L. Sparks (ed.), Methods of soil analysis, Page 3., Chemical methods-ASA and SSSA Book Series no. 5, Chp. 3, (pp 49–64.) Madison, WI.
Kesse, G. O. (1985). The mineral and rock resources of Ghana. Rotterdam, Boston: A. A Balkema.
Meili, M., Lindqvist, O., Johansson, K., Aastrup, M., Adersson, A., Bringmark, L., Hovsenius, G., Hankanson, L., Iverfeldt, A., & Timm, B. (1991). Mercury in the Swedish environment: recent research on causes, consequences and corrective methods. Water, Air, and Soil Pollution, 55, 1–261.
Nriagu, J. O. (1994). Mercury pollution from the past mining of gold and silver in the America. Science of the Total Environment, 149, 167.
Schuster, E. (1991). The behavior of mercury in the soil with special emphasis on complexation and absorption processes—a review of the literature; water. Air, Soil Pollution, 56, 667–680.
UNEP (2002): Report of the global mercury assessment working group on the work of its first Meeting, Geneva, Switzerland, September 2002
US EPA (1997). Environmental effects: fate and transport and ecological effects of mercury, http://www.epa.gov/mercury/eco.htm.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nartey, V.K., Klake, R.K., Doamekpor, L.K. et al. Speciation of mercury in mine waste: case study of abandoned and active gold mine sites at the Bibiani–Anwiaso–Bekwai area of South Western Ghana. Environ Monit Assess 184, 7623–7634 (2012). https://doi.org/10.1007/s10661-012-2523-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2523-2