Abstract
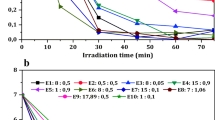
Laboratory photochemical experiments with stream water were done to characterize the photodegradation of dissolved organic carbon (DOC) and photochemical release of organically bound metals. The samples were collected from Bear Brook Watershed, Hadlock Brook, and Mud Pond Stream in Maine, USA, during January and April 2006. Filtered samples were irradiated in a reactor equipped with 350 nm irradiation lamps. Aliquots of irradiated samples were analyzed for DOC, dissolved aluminum (Ald) and iron (Fed), pH, and UV–Vis spectra. Organically bound metals (Feo and Alo) were measured after passing the sample through a column filled with a strong cation exchange resin (Dowex HCR-W2). UV radiation resulted in a decrease in DOC concentration and structural changes in DOC composition. UV–Vis spectra showed a decrease in aromaticity and molecular weight of DOC during irradiation. The DOC ranged from 0.1 to 0.35 mmol L − 1 at the beginning of experiments and decreased 5% to 37% after irradiation. Oxidation and structural changes in DOC resulted in the release of organically bound metals. Initial Feo concentrations ranged from 0.16 to 0.79 μmol L − 1 and decreased 56% to 81% during the irradiation. The concentration of Alo ranged from 1.0 to 3.85 μmol L − 1 and declined steadily throughout the irradiation, resulting in 8% to 76% decline. Degradation of a small percentage of organically bound Al and Fe occurs rapidly enough so as to be an important process in first- and second-order streams. Irradiation energy absorbed by samples during hours of laboratory experiments equates to days in stream environment. Degradation of more refractory complexes occurs on a time scale that requires longer residence times, such as in lakes. This study demonstrated a strong impact of photochemical degradation of DOC on its metal-complexing ability and capacity. The results also suggest different binding properties of Fe and Al in their organic complexes.
Similar content being viewed by others
References
Bertilsson, S., & Tranvik, L. J. (2000). Photochemical transformation of dissolved organic matter in lakes. Limnology and Oceanography, 45(4), 753–762.
Driscoll, C. T. (1984). A procedure for the fractionation of aqueous aluminum in dilute acidic waters. International Journal of Environmental Analytical Chemistry, 16, 267–284.
Emmenegger, L., Schönenberger, R., Sigg, L., & Sulzberger, B. (2001). Light-induced redox cycling of iron in circumneutral lakes. Limnology and Oceanography, 46, 49–61.
Frost, P. C., Larson, J. H., Kinsman, L. E., & Lamberti, G. A. (2005). Attenuation of ultraviolet radiation in streams of northern Michigan. Journal of the North American Benthological Society, 24(2), 246–255.
Gao, H., & Zepp, R. G. (1998). Factors influencing photoreactions of dissolved organic matter in a costal river of the Southeastern United States. Environmental Science & Technology, 32, 2940–2946.
Grant, H. R., Heisler, G. M., & Gao, W. (2002). Estimation of pedestrian level UV exposure under trees. Photochemistry and Photobiology, 75(4), 369–376.
Hatchard, C. G., & Parker, C. A. (1956). A new sensitive chemical actinometer. 2. Potassium ferrioxalate as a standard chemical. Proceedings of the Royal Society of London, A235, 518–536.
Howitt, J. A., Baldwin, D. S., Rees, G. N., & Hart, B. T. (2004). Facilitated heterogeneous photodegradation of dissolved organic matter by particulate iron. Environmental Chemistry, 1, 197–205.
Kieber, D. J., McDaniel, J., & Mopper, K. (1989). Photochemical source of biological substances in sea water: Implications for carbon cycling. Nature, 341, 637–639.
Köhler, S., Buffam, I., Jonsson, A., & Bishop, K. (2002). Photochemical and microbial processing of stream and soil water dissolved organic matter in a boreal forested catchment in northern Sweden. Aquatic Sciences, 64, 269–281.
Kopáček, J., Hejzlar, J., Kaňa, J., Porcal, P., & Klementová, Š. (2003). Photochemical, chemical, and biological transformations of dissolved organic carbon and its impact on alkalinity production in acidified lakes. Limnology and Oceanography, 48, 106–117.
Kopáček, J., Klementová, Š., & Norton, S. A. (2005). Photochemical production of ionic and particulate aluminum and iron in lakes. Environmental Science & Technology, 39, 3656–3662.
Kopáček, J., Marešová, M., Norton, S. A., Porcal, P., & Veselý, J. (2006). Photochemical source of metals for sediments. Environmental Science & Technology, 40, 4455–4459.
Larson, J. H., Frost, P. C., Lodge, D. M., & Lamberti, G. A. (2007). Photodegradation of dissolved organic matter in forested streams of the northern Great Lakes region. Journal of the North American Benthological Society, 26(3), 416–425.
Mantoura, R. F. C., Dickson, A., & Riley, J. P. (1978). The complexation of metals with humic materials in natural waters. Estuarine, Coastal and Marine Science, 6, 387–408.
Miles, C. J., & Brezonic, P. L. (1981). Oxygen consumption in humic-colored waters by photochemical ferrous–ferric catalytic cycle. Environmental Science & Technology, 15, 1089–1095.
Molot, L. A., & Dillon, P. J. (1997). Photolytic regulation of dissolved organic carbon in northern lakes. Global Biogeochemical Cycles, 11, 357–365.
Molot, L. A., Hudson, J. J., Dillon, P. J., & Miller, S. A. (2005). Effect of pH on photo-oxidation of dissolved organic carbon by hydroxyl radicals in a coloured, softwater stream. Aquatic Sciences, 67, 189–195.
Mopper, K., & Zhou, X. (1990). Hydroxyl radical photoproduction in the sea and its potential impact on marine processes. Science, 250, 661–664.
Moran, M. A., & Zepp, R. G. (1997). Role of photoreactions in the formation of biologically labile compounds from dissolved organic matter. Limnology and Oceanography, 42, 1307–1316.
Norton, S. A., Kahl, J., Fernandez, I., Haines, T., Rustad, L., Nodvin, S., et al. (1999). The Bear Brook Watershed, Maine (BBWM), USA. Environmental Monitoring and Assessment, 55, 7–51.
Peuravuori, J., & Pihlaja, K. (1997). Molecular size distribution and spectroscopic properties of aquatic humic substances. Analytica Chimica Acta, 337, 133–149.
Powell, A. T., & Wilson-Finelli, A. (2003). Photochemical degradation of organic iron complexing ligands in seawater. Aquatic Sciences, 65, 367–374.
Rose, A. L., & Waite, T. D. (2005). Reduction of organically complexed ferric iron by superoxide in a simulated natural water. Environmental Science & Technology, 39, 2645–265.
Shank, G. C., Whitehead, R. F., Smith, M. L., Skrabal, S. A., & Kieber, R. J. (2006). Photodegradation of strong copper-complexing ligands in organic-rich estuarine waters. Limnology and Oceanography, 51, 884–892.
Shiller, A. M., Duan, S., van Erp, P., & Bianchi, T. S. (2006). Photo-oxidation of dissolved organic matter in river water and its effect on trace element speciation. Limnology and Oceanography, 51, 1716–1728.
Sl’awinskij, J., Puzyna, W., & Sl’awinska, D. (1978). Chemiluminescence in photooxidation of humic acids. Photochemistry and Photobiology, 28, 75–81.
Vance, G. F., Stevenson, F. J., & Sikora, F. J. (1996). Environmental chemistry of aluminum–organic complexes. In G. Sposito (Ed.), The environmental chemistry of aluminum (pp. 169–220). Chelsea: Lewis.
Weishaar, J. L., Aiken, G. R., Bergamashi, B. A., Fram, M. S., Fujii, R., & Mopper, K. (2003). Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environmental Science & Technology, 37, 4702–4708.
Xie, H., Zafiriou, O. C., Cai, W.-J., Zepp, R. G., & Wang, Y. (2004). Photooxidation and its effects on the carboxyl content of dissolved organic matter in two coastal rivers in the southeastern United States. Environmental Science & Technology, 38, 4113–4119.
Zepp, R. G., Faust, B. C., & Hoigné, J. (1992). Hydroxyl radical formation in aqueous reaction (pH 3–8) of iron(II) with hydrogen peroxide: the photo-Fenton reaction. Environmental Science & Technology, 26, 313–319.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Porcal, P., Amirbahman, A., Kopáček, J. et al. Experimental photochemical release of organically bound aluminum and iron in three streams in Maine, USA. Environ Monit Assess 171, 71–81 (2010). https://doi.org/10.1007/s10661-010-1529-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-010-1529-x