Abstract
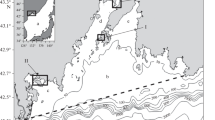
The contents of heavy metals (Fe, Mn, Pb, Cu, Cd, and Hg) dissolved in water and suspended solids of Gökova Bay—partly and fully sampled in 2005 and 2006, respectively—are quite higher than the average values encountered in uncontaminated sea water. The high concentrations are associated with terrestrial inputs from the mining zones and anthropogenic (domestic + industrial) sources. Moreover, the distribution of Fe and Cu is affected by primary production because these elements function as nutrients in biological activities. The Cr, Ni, and Fe concentrations of surface sediments are above the shale average. The Cr and Ni contents of surface sediments representative of river mouths strongly correlate with total phosphorus contents. In a sulfide-poor environment, Pb and Cu were concentrated at a higher ratio in surface sediments than Cd, probably due to higher stabilities of their surface complexes with amorphous iron oxides and clay minerals existing as major components in the sediments. The exceptional enrichment of Zn may be attributed to double oxide formation with amorphous iron oxides in sediments. The high metal values are most probably caused by terrestrial inputs from anthropogenic sources and the mining zones at the southeast part of the bay. The Al, Mn, Pb, Cu, Zn, and Hg contents are below the shale average. The low values have possibly originated from the coarse-grained sandy sediments having a low affinity for metals. There are no distinct differences in the metal distributions in water and suspended matter between the years 2005 and 2006 in the bay, probably due to low sedimentation rates.
Similar content being viewed by others
References
Allen, H. E., & Minear, R. A. (1982). Metallic ions. In M. J. Suess (Ed.), Examination of water for pollution control (chapter 2). A reference handbook (Vol. 2, pp. 124–168). New York: Pergamon.
APHA, AWWA, WPCF (1980). Standard methods for the examination of water and wastewater (17th ed). Washington, D.C.: American Public Health Association.
Baba, A., Kaya, A., & Birsoy, Y. K. (2003). The effect of Yatağan thermal power plant (Muğla, Turkey) on the quality of surface and groundwaters. Water, Air and Soil Pollution, 149(1–4), 93–111.
Boughriet, A., Water, M., Cordier, C., Douez, C., Deram, I., Marchin, E., et al. (1994). Chemical Speciation of some particulate elements in the English Channel and impact of human activities on the magnetic behaviour of suspended matter. Marine Pollution Bulletin, 28(9), 541–556.
Bruland, K. W., Coale, K. H., & March, L. (1985). Analysis of seawater for dissolved cadmium, copper and lead: An inter comparison of voltametric and atomic adsorption methods. Marine Chemistry, 17, 285–300.
Bruland, K. W., Franks, R. P., Knauer, G. A., & Marchin, J. H. (1979). Sampling and analytical methods for the determination of copper, cadmium, zinc and nickel at the nanogram per liter level in seawater. Analytica Chimica Acta, 105, 233–245.
Folger, D. W. (1972). Texture and organic carbon content of bottom sediments in some estuaries of the United States. In B. W. Nelson (Ed.), Environmental framework of coastal plain estuaries (pp. 391–408). Boulder: Geological Society of America Memoir.
Filipek, H. L., & Owen, R. M. (1978). Geochemical associations and grain size partitioning of heavy metals in lacustrine sediments. Chemical Geology, 26, 105–117.
Hornberger, M. I., Luoma, S. N., van Geen, A., Fuller, C., & Anima, R. (1999). Historical trends of metals in the sediments of San Fransisco Bay, California. Marine Chemistry, 64, 39–55.
Horowitz, A. J., Elrick, K. A., & Hooper, R. P. (1989). The prediction of aquatic sediment-associated trace element concentrations using readily obtainable geochemical factors. In Proceedings of the international conference on heavy metals in the environment (pp. 493–496).
Krauskopf, K. B. (1979). Introduction to geochemistry. International series in the earth and planetary scienes (pp. 544–545). Tokyo: McGraw-Hill.
Landing, W. M., & Lewis, B. L. (1991). Collection processing and analysis of marine particulate and colaidal material for transition metals. Marine particles: Analysis and characterization geophysical monograph 63. American Geophysical Union.
Loring, D. H., & Rantala, R. T. T. (1992). Manual for the geochemical analyses of marine sediments and suspended particulate matter. Earth Science Review, 32, 235–283.
Pekey, H., Karakaş, D., Ayberk, S., Tolun, L., & Bakoğlu, M. (2004). Ecological risk assessment using trace elements from surface sediments of Izmit Bay (Northeastern Marmara Sea) Turkey. Marine Pollution Bulletin, 48, 946–953.
Perin, G., Fabris, R., Manente, S., Wagener, A. R., Hamacher, C., & Scotto, S. (1997). A five-year study on the heavy-metal pollution of Guanabara Bay sediments (Rio de Jenerio, Brazil) and evaluation of the metal bioavailability by means of geochemical speciation. Water Research, 31, 3017–3028.
Pulford, I. D., & Bakhsh, A. (1989). Uptake of zinc by soil: Adsorption or precipitation? In Proceedings of the international conference on heavy metals in the environment (Vol. 2, pp.193–196).
Riley, J. P., Chester, R., & Skirrow, G. (1971). Introduction to marine chemistry. New York: Academic.
Sing, J., Agrawal, M., & Narayan, D. (1995). Changes in soil characteristic around coal-fired power plant. Environmental International, 21(1), 93–102.
Tuna, L. A., Yağmur, B., Hakerlerler, H., Kılınç, R., Yokas, İ., & Bürün, B. (2005). Muğla Bölgesi’ndeki Termik Santrallerinden Kaynaklanan Kirlik Üzerine Araþtırmalar. Uğla Üniversirtesi, Bilimsel Araþtırma Projesi-Kesin Paporu.
Zhang, J., & Liu, C. L. (2002). Riverine composition and estuarine geochemistry of particulate metals in China—Weathering features, anthropogenic impact and chemical fluxes. Estuarine, Coastal and Shelf Science, 54, 1051–1070.
Zhang, L., Ye, X., Feng, H., Jing, Y., Ouyang, T., Yu, X., et al. (2007). Heavy metal contamination in western Xiamen Bay sediments and its vicinity. China, Marine Pollution Bulletin, 54, 974–982.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balkis, N., Aksu, A., Okuş, E. et al. Heavy metal concentrations in water, suspended matter, and sediment from Gökova Bay, Turkey. Environ Monit Assess 167, 359–370 (2010). https://doi.org/10.1007/s10661-009-1055-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-009-1055-x