Abstract
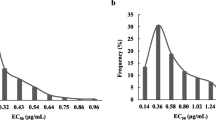
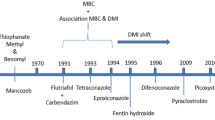
Cercospora beticola Sacc. is the most destructive pathogen of sugar beet (Beta vulgaris L.) and causes Cercospora leaf spot (CLS). Since 1986, fungicides that function as demethylation inhibitors (DMIs) have been used to control CLS in Hokkaido, which is the only area in Japan where sugar beet is grown. Reduced sensitivity of C. beticola to DMI fungicides, based on the half maximal effective concentration (EC50), was first reported in Hokkaido in 1999, however the fungicides continued to be used effectively until 2014. In a field experiment in 2016, we found that the efficacy of difenoconazole against the field population of C. beticola was greatly reduced. We subsequently tested over 600 isolates collected throughout the sugar beet-growing region of Hokkaido and revealed that the mean resistance factor of four DMI fungicides (difenoconazole, fenbuconazole, tebuconazole, and tetraconazole) were high, which indicates that DMI-resistant isolates were distributed throughout the beet cultivation area. Moreover, we identified three types of isolates that have unique cross-resistance patterns between difenoconazole and fenbuconazole, with their EC50 rate (= difenoconazole EC50/ fenbuconazole EC50) converged to 31, 4.0, and 0.40, respectively, which appeared to be affected by the local history of fungicide usage. The F144L substitution in CbCYP51 was only found in the group whose EC50 rate was 0.40. This is the first report of DMI resistance in C. beticola in Japan, and the findings in this study could contribute to our understanding of the mechanism of DMI resistance.




Similar content being viewed by others
Change history
12 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10658-021-02223-w
References
Bolton, M. D., Birla, K., Rivera-Varas, V., Rudolph, K. D., & Secor, G. A. (2012). Characterization of CbCyp51 from field isolates of Cercospora beticola. Phytopahol, 102, 298–305.
Cañas-Gutiérrez, G. P., Angarita-Velásquez, M. J., Restrepo-Flórez, J. M., Rodríguez, P., Moreno, C. X., & Arango, R. (2009). Analysis of the CYP51 gene and encoded protein in propiconazole-resistant isolates of Mycosphaerella fijiensis. Pest Management Science, 65, 892–899.
Chikuo, Y., Sugimoto, T., Kanzawa, K., & Uchino, H. (1984). Occurrence of Kasugamycin-resistant strains of Cercospora beticola in sugar beet. Ann Phytopath Soc Japan, 50, 637–640.
Clement, M., Snell, Q., Walke, P., Posada, D., Crandall, K. (2002) TCS: Estimating gene genealogies. Proc 16th Int Parallel Distrib Process Symp 2:184.
Cools, H. J., & Fraaije, B. A. (2013). Update on mechanisms of azole resistance in Mycosphaerella graminicola and implications for future control. Pest Management Science, 69, 150–155.
Cools, H. J., Hawkins, N. J., & Fraaije, B. A. (2013). Constraints on the evolution of azole resistance in plant pathogenic fungi. Plant Pathology, 62, 36–42.
Fraaije, B. A., Cools, H. J., Motteram, J., Clark, W. S., & Lucas, J. A. (2007). A novel substitution I381V in the sterol 14 α-demethylase (CYP51) of Mycosphaerella graminicola is differentially selected by azole fungicides. Molecular Plant Pathology, 8, 245–254.
FRAC (Fungicide Resistance Action Committee) (2019) FRAC pathogen risk list. https://www.frac.info/docs/default-source/publications/pathogen-risk/frac-pathogen-list-2019.pdf. Accessed 2020.
FRAC (Fungicide Resistance Action Committee) (2018) FRAC statement on multisite fungicides. https://www.frac.info/docs/default-source/publications/statement-on-multisite-fungicides/frac-statement-on-multisite-fungicides-2018.pdf?sfvrsn=3c25489a_2. Accessed 2020.
Frankel, O., Cadle-Davidson, L., Wilcox, W. F., & Milgroom, M. G. (2015). Mechanizms of resistance to an azole fungicide in the grapevine powdery mildew fungus, Erisiphe necator. Phytopathol, 105, 370–377.
Hellin, P., King, R., Urban, M., Hammond-Kosack, K. E., Legrève, A. (2018) The adaptation of Fusarium culmorum to DMI fungicides is mediated by major transcriptome modifications in response to azole fungicide, including the overexpression of a PDR transporter (FcABC1). Frontiers in Microbiol 9: (1385).
Hermann, D., & Stenzel, K. (2019). FRAC mode-of-action classification and resistance risk of fungicides. In P. Jeschke, M. Witschel, W. Krämer, & U. Schimer (Eds.), Modern crop protection compounds (pp. 589–608). Mannheim: Wiley.
Hikota, T., Hirata, T., Hirata, A., Uchino, H., & Watanabe, H. (1999). Efficacy of difenoconazole against Cercospora leaf spot on sugar beet (abstract in Japanese). Jpn J Phytopathol, 65, 403.
Horita, H., Yasuoka, S., & Abe, H. (1996). Disease spread of Cercospora leaf spot in sugar beet and the use of proportion of disease plant as monitoring method (in Japanese). Hokunou, 63, 78–85.
Iketani, M. S., & Iketani, S. (2015). Concept for control of Cercospora leaf spot of sugar beet to cope with climate change (abstract in Japanese). Jpn J Phytopathol, 81, 93.
Iketani, S., Ohnami, M., & Yamazaki, H. (2016). A new sugar beet variety ‘Angy’ (in Japanese). Bulletin of Hokkaido Reser Org Agri Exper Stat, 100, 77–81.
Jacobson, B. J., Franc, G. D. (2009) Cercospora leaf spot. In: Harveson, R. M., Hanson, L. E., Hein, G. L. (Ed.), Compendium of Beet disease and pests, 2nd edn (pp. 7–10). American phytopathological society.
Karaoglanidis, G. S., Ioannidis, P. M., & Thanasoulopoulos, C. C. (2000). Reduced sensitivity of Cercospora beticola isolates to sterol-demethylation-inhibiting fungicides. Plant Pathology, 49, 567–572.
Karaoglanidis, G. S., & Thanasoulopoulos, C. C. (2003). Cross-resitance patterns among sterol biosynthesis inhibiting fungicides (SBIs) in Cercospora beticola. European Journal of Plant Pathology, 109, 929–934.
Kayamori, M., Sasaki, J., Matsui, R., Shinmura, A., Horita, H., & Satou, M. (2012). First report of downy mildew of carnation caused by Peronospora dianthicola in Japan. Journal of General Plant Pathology, 78, 364–367.
Kayamori, M., Shimizu, M., Yamana, T., Komatsu, T., Iketani, M. S., Shinmura, A., Sasaki, J., Kozawa, T., Notsu, A., & Yasuoka, S. (2020). First report of QoI resistance in Cercospora beticola in sugar beet in Japan. Journal of General Plant Pathology, 86, 149–153.
Khan, J., del Rio, L. E., Nelson, R., Rivera-Varas, V., & Secor, G. A. (2008). Survival, dispersal, ad primary infection site for Cerocospora beticola in sugar beet. Plant Disease, 92, 741–745.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549.
Kondo, Y., Sato, Y., Adachi, T., Otake, M., & Saito, H. (2016). Characteristics of new sugar beet variety “2K314” (in Japanese). Proc Japan Soc Sugar Beet Technol, 57, 23–24.
Leigh, J. W., & Bryant, D. (2015). PopART: Full-feature softwawre for haplotype network construction. Methods in Ecology and Evolution, 6, 1110–1116.
Ma, Z., Proffer, T. J., Jacobs, J. L., & Sundin, G. W. (2006). Overexpression of the 14α-demethylase target gene (CYP51) mediates fungicide resistance in Blumeriella jaapii. Applied and Environmental Microbiology, 72, 2581–2585.
Mullins, J. G. L., Parker, J. E., Cools, H. J., Togawa, R. C., Lucas, J. A., Fraaife, B. A., Kelly, D. E., & Kelly, S. L. (2011). PLoS ONE, 6(6), e20973. https://doi.org/10.1371/journal.pone.0020973.
Rangel, L. I., Spanner, R. E., Ebert, M. K., Pethybridge, S. J., Stukenbrock, E. H., Jonge, R., Secor, G. A., & Bolton, M. D. (2020). Cercospora beticola: The intoxicating lifestyle of the leaf spot pathogen of sugar beet. Molecular Plant Pathology, 21, 1020–1041.
Secor, G., Rivera, V. V., Khan, M. F. R., & Gudmestad, N. C. (2010). Monitoring fungicide sensitivity of Cercospora beticola of sugar beet for disease management decisions. Plant Disease, 94, 1272–1282.
Secor, G., Rivera, V., Bolton, M. (2012) Sensitivity of Cercospora beticola to foliar fungicides in 2011. 2011 Sugarbeet Res. Ext. Rep. N. D. State Univ. Fargo: Online.
Secor, G., Rivera, V., Khan, M. (2019) Sensitivity of Cercospora beticola to foliar fungicides in 2018. 2018 Sugarbeet Res Ext Rep N D State Univ Fargo 49:191-198.
Shimizu, M. (2007). Decreased sensitivity to DMI-fungicide in Cercospora beticola, the causal fungus of Cercospora leaf spot on sugar beet (in Japanese). Plant Protection, 61, 421–425.
Shrestha, S., Neubauer, J., Spanner, R., Natwick, M., Rios, J., Metz, N., Secor, G. A., & Bolton, M. D. (2020). Rapid detection of Cercospora beticola in suger beet and mutations associated with fungicide resistance using LAMP or probe-based qPCR. Plant Disease, 104, 1654–1661.
Spanner, R., Taliadoros, D., Richards, J., Rivera-Varas, V., Neubauer, J., Natwick, M., Hamilton, O., Vaghefi, N., Pethybridge, S., Secor, G. A., Friesen, T. L., Stukenbrock, E. H., Bolton, M. D. (2020) Genome-wide association studies reveal the complex genetic architecture of DMI fungicide resistance in Cercospora beticola. bioRxiv preprint https://doi.org/10.1101/2020.11.12.379818
Trkulja, N., Milosavljević, A., Atanisavljevic, R., Mitrović, M., Jović, J., Toševski, I., & Bošković, J. (2015). Occurrence of Cercospora beticola populations resistant to benzimidazoles and demethylation-inhibiting fungicides in Serbia and their impact on disease management. Crop Protection, 75, 80–87.
Trkulja, N. R., Milosavljević, A. G., Mitrović, M. S., Jović, J. B., Toševski, I. T., Khan, M. F. R., & Secor, G. A. (2017). Molecular and experimental evidence of multi-resistance of Cercospora beticola field populations to MBC, DMI and QoI fungicides. European Journal of Plant Pathology, 149, 895–910.
Trueman, C. L., Hanson, L. E., Somohano, P., & Rosenzweig, N. (2017). First report of DMI-insensitive Cercospora beticola on sugar beet in Ontario, Canada. New Disease Reports, 36, 20.
Uchino, H., & Kanzawa, K. (1991). Application of a new fungicide “Difenoconazole” to control of Cercospora and Ramularia leaf spot of sugar beet in Hokkaido (in Japanese with English summary). Proc Japan Soc Sugar Beet Technol, 33, 88–96.
Uchino, H., Kanzawa, K., & Yamakami, M. (1992). The occurrence of Cercospora leaf spot of sugar beet and climatic factors influencing its spread in Tokachi district (in Japanese with English summary). Proc Japan Soc Sugar Beet Technol, 34, 117–126.
Uchino, H., & Watanabe, H. (1998). Controlling Cercospora leaf spot of sugar beet by applying tetraconazole (in Japanese with English summary). Proc Japan Soc Sugar Beet Technol, 40, 80–84.
Uchino, H., Hikota, T., Watanabe, H., & Kanzawa, K. (1999). Sensitivity to difenoconazole and thiophanate-methyl of Cercospora beticola isolated from a sugar beet field after successive treatment by both chemicals (abstract in Japanese). Jpn J Phytopathol, 65, 403.
Acknowledgments
We thank Ms. M. Nagahama, HRO Kamikawa Agricultural Experiment Station, Staffs of Nitten Sugar Beet Mfg. Co., Ltd., Hokkaido Sugar Co., Ltd., and Hokuren Federation of Agricultural Cooperatives; the staff of each agricultural extension center in Hokkaido for collecting leaves with CLS; Dr. T. Komatsu, Dr. M. S. Iketani, Mr. J. Sasaki, Mr. T. Kozawa and Mr. S. Yasuoka, Hokkaido Research Organization, for isolating Cercospora beticola; Ms. S. Yamashita, lab assistant of HRO Tokachi Agricultural Experiment Station, for help especially in determining EC50 values on media. Special thanks to Mr. M. Shimizu and Mr. S. Yasuoka, HRO Kitami Agricultural experiment Station, for helpful advice. We thank Dr. Y. Kawabata of TUAT for help in special arrangements for the one of the authors’ stay in Japan. We thank for the Special Research Fund of the Institute of Global Innovation Research at Tokyo University of Agriculture and Technology (GIR-TUAT), Japan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
This paper does not contain studies on human or animal participants.
Informed consent
The paper has not been submitted elsewhere for publication, in whole or in part.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Supplementary Information
ESM 1
(XLSX 42 kb)
Rights and permissions
About this article
Cite this article
Kayamori, M., Zakharycheva, A., Saito, H. et al. Resistance to demethylation inhibitors in Cercospora beticola, a pathogen of sugar beet in Japan, and development of unique cross-resistance patterns. Eur J Plant Pathol 160, 39–52 (2021). https://doi.org/10.1007/s10658-021-02219-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-021-02219-6