Abstract
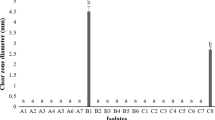
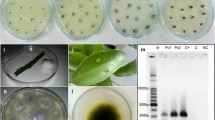
The relative susceptibility of six Rutaceae family genotypes to bacterial citrus canker (CC) caused by Xanthomonas citri subsp. citri (Xcc) was assessed by measuring and comparing in planta bacterial populations over time following inoculation using a minimally destructive inoculation. These genotypes included lime (Citrus aurantifolia), grapefruit (C. paradisi), sweet orange (C. sinensis), calamondin (C. reticulata X C. japonica), kumquat (C. japonica), and orange jessamine (Murraya paniculata). Internal Xcc populations in orange jessamine plateaued early and remained significantly lower than other genotypes. Xcc populations increased rapidly in all other genotypes, decreasing precipitously and significantly over time in kumquat and calamondin as compared to the more susceptible genotypes sweet orange, grapefruit, and lime. Xcc populations in calamondin peaked by 7 DPI then began to fall significantly relative to the more susceptible genotypes sweet orange, grapefruit, and lime. Given the steep decline in populations in calamondin and kumquat, we compared water-soluble extracts from healthy leaf tissue from all previously investigated Citrus genotypes to determine if the extracts had any inhibitory effect on Xcc, implicating them in this abrupt population decline late in the infection process. Only extracts from calamondin leaf tissue significantly inhibited Xcc growth within 24 h. Potentially, constitutively produced inhibitory compounds in calamondin may result in less severe CC symptoms as a result of the rapid decline in Xcc populations.




Similar content being viewed by others
References
Bittancourt, A. A. (1957). O Cancro citrico. O Biologico, 23, 101–111.
Bock, C. H., Parker, P. E., & Gottwald, T. R. (2005). Effect of simulated wind-driven rain on duration and distance of dispersal of Xanthomonas axonopodis pv. citri from canker-infected citrus trees. Plant Disease, 89(1), 71–80. https://doi.org/10.1094/Pd-89-0071.
Chen, P. S., Wang, L. Y., Chen, Y. J., Tzeng, K. C., Chang, S. C., Chung, K. R., et al. (2012). Understanding cellular defence in kumquat and calamondin to citrus canker caused by Xanthomonas citri subsp. citri. Physiological and Molecular Plant Pathology, 79, 1–12. https://doi.org/10.1016/j.pmpp.2012.03.001.
Del Rio, J., Gomez, P., Baidez, A., Arcas, M., Botia, J., & Ortuno, A. (2004). Changes in the levels of polymethoxyflavones and flavanones as part of the defense mechanism of Citrus sinensis (Cv. Valencia late) fruits against Phytophthora citrophthora. Journal of Agricultural and Food Chemistry, 52, 1913–1917.
Dopson, R. N. (1964). The eradication of citrus canker. Plant Disease Report, 48, 30–31.
Ference, C. M. (2016). The production and accumulation of phenolic compounds in response to infection by Xanthomonas citri subsp. citri in leaves of citrus genotypes with reduced susceptibility to citrus canker. Unpublished doctoral dissertation, University of Florida, Gainesville.
Francis, M. I., Kostenyuk, I., Orbovic, V., Loskutov, A., Zolotukhin, M., & Graham, J. (2011). Automated needle-free injection method for delivery of bacterial suspensions into citrus leaf tissues. Journal of Phytopathology, 159, 347–351.
Gochez, A. M., Shantharaj, D., Potnis, N., Zhou, X., Minsavage, G. V., White, F. F., et al. (2016). Molecular characterization of xopAG effector-avrGf2 from Xanthomonas fuscans subsp. aurantifolii in grapefruit. Molecular Plant Pathology, 18, 405–419. https://doi.org/10.1111/mpp.12408.
Gottwald, T. R., & Graham, J. H. (1992). A device for precise and nondisruptive stomatal inoculation of leaf tissue with bacterial pathogens. Phytopathology, 82(9), 930–935. https://doi.org/10.1094/Phyto-82-930.
Gottwald, T. R., Alvarez, A. M., Hartung, J. S., & Benedict, A. A. (1991). Diversity of Xanthomonas campestris pv citrumelo strains associated with epidemics of citrus bacterial spot in Florida citrus nurseries: Correlation of detached leaf, monoclonal-antibody, and restriction-fragment-length-polymorphism assays. Phytopathology, 81(7), 749–753. https://doi.org/10.1094/Phyto-81-749.
Gottwald, T. R., Graham, J. H., Civerolo, E. L., Barrett, H. C., & Hearn, C. J. (1993). Differential host-range reaction of citrus and citrus relatives to citrus canker and citrus bacterial spot determined by leaf mesophyll susceptibility. Plant Disease, 77(10), 1004–1009.
Gottwald, T. R., Bassanezi, R. B., Amorim, L., & Bergamin-Filho, A. (2007). Spatial pattern analysis of citrus canker-infected plantings in Sao Paulo, Brazil, and augmentation of infection elicited by the Asian leafminer. Phytopathology, 97(6), 674–683. https://doi.org/10.1094/Phyto-97-6-0674.
Graham, J. H., Gottwald, T. R., Cubero, J., & Achor, D. S. (2004). Xanthomonas axonopodis pv. citri: Factors affecting successful eradication of citrus canker. Molecular Plant Pathology, 5(1), 1–15. https://doi.org/10.1046/J.1364-3703.2003.00197.X.
Jiao, H. J., Wang, S. Y., & Civerolo, E. L. (1992). Enzymatic activities of citrus leaves from plants resistant and susceptible to citrus bacterial canker disease. Environmental and Experimental Botany, 32(4), 463–470.
Lattanzio, V., Lattanzio, V. M. T., & Cardinali, A. (2006). Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In F. Imperato (Ed.), Phytochemistry: Advances in research (pp. 23–67). Kerala: Research Signpost.
Lee, H. A. (1918). Further data on the susceptibility of Rutaceous plants to citrus-canker. Journal of Agricultural Research, 15, 0661–0666.
Lee, H. A. (1921). The increase in resistance to citrus canker with the advance in maturity of citrus trees. Phytopathology, 11(2), 70–73.
Luthra, J. C., & Sattar, A. (1942). Citrus canker and its control in Punjab. Punjab Fruit Journal, 6(1), 179–182.
Manthey, J. A., & Narciso, J. A. (2011). Detection of fluorescent compounds in citrus leaf cankers. Proceedings of the Florida State Horticultural Society, 124, 227–231.
Manthey, J. A., & Narciso, J. A. (2013). The HPLC-fluorescence detection of coumarins in ‘Hamilin’ sweet orange and ‘Marsh’ grapefruit leaf cankers. Proceedings of the Florida State Horticultural Society, 126, 217–219.
Mysore, K. S., & Ryu, C. M. (2004). Nonhost resistance: How much do we know? Trends in Plant Science, 9(2), 97–104. https://doi.org/10.1016/j.tplants.2003.12.005.
Palm, M. E., & Civerolo, E. (1994). Isolation, pathogenicity, and partial host range of Alternaria limicola, causal agent of mancha foliar de los citricos in Mexico. Plant Disease, 78, 879–883.
Pruvost, O., Magne, M., Boyer, K., Leduc, A., Tourterel, C., Drevet, C., et al. (2014). A MLVA genotyping scheme for global surveillance of the citrus pathogen Xanthomonas citri pv. citri suggests a worldwide geographical expansion of a single genetic lineage. PLoS One, 9(6), e98129. https://doi.org/10.1371/journal.pone.0098129.
Reymond, P., Weber, H., Damond, M., & Farmer, E. E. (2000). Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell, 12(5), 707–719. https://doi.org/10.1105/tpc.12.5.707.
Rybak, M., Minsavage, G. V., Stall, R. E., & Jones, J. B. (2009). Identification of Xanthomonas citri ssp citri host specificity genes in a heterologous expression host. Molecular Plant Pathology, 10(2), 249–262. https://doi.org/10.1111/j.1364-3703.2008.00528.x.
Schaad, N. W., Postnikova, E., Lacy, G., Sechler, A., Agarkova, I., Stromberg, P. E., Stromberg, V. K., & Vidaver, A. K. (2005). Reclassification of Xanthomonas campestris pv. citri (ex Hasse 1915) Dye 1978 forms A, B/C/D, and E as X. smithii subsp. citri (ex Hasse) sp. nov. nom. rev. comb. nov., X. fuscans subsp. aurantifolii (ex Gabriel 1989) sp. nov. nom. rev. comb. nov., and X. alfalfae subsp. citrumelo (ex Riker and Jones) Gabriel et al., 1989 sp. nov. nom. rev. comb. nov.; X. campestris pv. malvacearum (ex Smith 1901) Dye 1978 as X. smithii subsp. smithii nov. comb. nov. nom. nov.; X. campestris pv. alfalfae (ex Riker and Jones, 1935) Dye 1978 as X. alfalfae subsp. alfalfae (ex Riker et al., 1935) sp. nov. nom. rev.; and “var. fuscans” of X. campestris pv. phaseoli (ex Smith, 1987) Dye 1978 as X. fuscans subsp. fuscans sp. nov. Systematic and Applied Microbiology, 28(6), 494–518.
Schoulties, C. L., Civerolo, E., Miller, J. W., Stall, R. E., Krass, C. J., Poe, S. R., et al. (1987). Citrus canker in Florida. Plant Disease, 71(5), 388–395.
Schubert, T. S., Rizvi, S. A., Sun, X., Gottwald, T. R., Graham, J. H., & Dixon, W. N. (2001). Meeting the challenge of eradicating citrus canker in Florida—again. The American Phytopathological Society, 85(4), 340–356.
Sun, X. A., Stall, R. E., Jones, J. B., Cubero, J., Gottwald, T. R., Graham, J. H., et al. (2004). Detection and characterization of a new strain of citrus canker bacteria from key Mexican lime and alemow in South Florida. Plant Disease, 88(11), 1179–1188. https://doi.org/10.1094/Pdis.2004.88.11.1179.
Treutter, D. (2006). Significance of flavonoids in plant resistance: A review. Environmental Chemistry Letters, 4, 147–157. https://doi.org/10.1007/s10311-006-0068-8.
Verniere, C., Hartung, J. S., Pruvost, O., Civerolo, E., Alvarez, A. M., Maestri, P., et al. (1998). Characterization of phenotypically distinct strains of Xanthomonas axonopodis pv. citri from Southwest Asia. European Journal of Plant Pathology, 104, 477–487.
Wang, Y., Fu, X. Z., Liu, J. H., & Hong, N. (2011). Differential structure and physiological response to canker challenge between ‘Meiwa’ kumquat and ‘Newhall’ navel orange with contrasting resistance. Scientia Horticulturae, 128, 115–123.
Yang, L., Ding, W., Xu, Y., Wu, D., Li, S., Chen, J., & Guo, B. (2016). New insights into the antibacterial activity of hydroxycoumarins against Ralstonia solanacearum. Molecules, 21(4), 468. https://doi.org/10.3390/molecules21040468.
Acknowledgements
The authors would like to thank Timothy Gottwald, Greg McCollum, and Xiuxiu Sun for their assistance. Facilities and funding supplied by the United States Department of Agriculture, Agricultural Research Service, United States Horticultural Research Laboratory, Fort Pierce, Florida, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
No human and/or animal participants were involved in this research.
Informed consent
All authors consent to this submission.
Rights and permissions
About this article
Cite this article
Ference, C.M., Baldwin, E.A., Manthey, J.A. et al. Inhibitory extracts of calamondin leaves associated with precipitous decline of Xanthomonas citri subsp. citri populations. Eur J Plant Pathol 156, 451–461 (2020). https://doi.org/10.1007/s10658-019-01894-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01894-w