Abstract
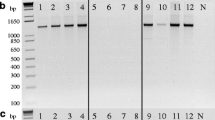
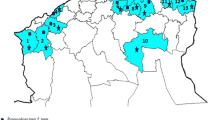
Pyrenophora graminea is the main fungal species associated with barley leaf stripe disease worldwide. Even though a heterothallic mating strategy has been proven for P. graminea, this species is mainly known based on the asexual morph in nature and there is no available information on the prevalence of an active sexual cycle within the populations of this species in Iran as well as many other barley-producing countries. The feasibility of a cryptic sexual cycle within Iranian isolates of P. graminea was assessed through analyzing the distribution and frequency of the mating type alleles on micro-spatial and macro-geographical scales. A total number of 306 P. graminea isolates were obtained from 93 fields in 45 geographical regions across East Azerbaijan province during 2016–2017. A multiplex PCR assay was developed for simultaneous identification of P. graminea and screening of its mating type alleles using previously designed primer sets. Using the multiplex PCR assay, a 435-bp band was consistently amplified from all P. graminea isolates; while a 1300-bp fragment or a 1150-bp fragment was only amplified from the isolates harboring MAT-1 and MAT-2 alleles, respectively. The mating type identity of 164 isolates was determined as MAT-1 and 142 isolates as MAT-2. Results of the present study revealed a nearly equal distribution (1:1 ratio; X2=1.582) of mating type alleles within and between different populations of P. graminea. Results of the micro-spatial and macro-geographical distribution of mating types showed that both mating types were often present on almost all studied scales, including: within the same lesion of each leaf from a single barley plant, from the same field or different fields in the same region, and from different regions. Based on the results of the current study and referring to the earlier reports on the population structure of P. graminea, it is concluded that this pathogen undergoes regular cycles of sexual recombination in most of the examined regions.


Similar content being viewed by others
References
Ahmadi, K., Golizadeh, H., Ebadzadeh, H. R., Hoseynpoor, R., Abdshah, H., Kazemian, A., & Rafiei, M. (2016). Agricultural statistics of crop year 2014–2015. Tehran: Ministry of Agriculture Jihad (in Persian).
Akhavan, A., Turkington, T. K., Kebede, B., Tekauz, A., Kutcher, H. R., Kirkham, C., Xi, K., Kumar, K., Tucker, J. R., & Strelkov, S. E. (2015). Prevalence of mating type idiomorphs in Pyrenophora teres f. teres and Pyrenophora teres f. maculata populations from the Canadian prairies. Canadian Journal of Plant Pathology, 37, 52–56.
Al-Daoude, A., Arabi, M. I. E., Nabulsi, I., & MirAli, N. (2012). Molecular phylogeny of Pyrenophora graminea as determined by RAPD and ISSR fingerprints. Journal of Plant Biology Research, 1(2), 25–35.
Antolin, M. F., Ode, P. J., Heimpel, G. E., O'Hara, R. B., & Strand, M. R. (2003). Population structure, mating system, and sex determining allele diversity of the parasitoid wasp Habrobracon hebetor. Heredity, 91, 373–381.
Arabi, M. I. E., & Jawhar, M. (2004). Genetic variation among Syrian Pyrenophora graminea isolates as determined by protein profile analysis. Advances in Horticulyural Science, 18, 132–137.
Arabi, M. I. E., & Jawhar, M. (2011). Vegetative compatibility groups and virulence variation among isolates of Pyrenophora graminea. The Plant Pathology Journal, 27(2), 116–119.
Arabi, M. I. E., Jawhar, M., & MirAli, N. (2006). Polypeptide patterns of Syrian isolates of Pyrenophora graminea. Journal of Plant Pathology, 88(2), 157–160.
Ariyawansa, H. A., Kang, J. C., Alias, S. A., Chukeatirote, E., & Hyde, K. D. (2014). Pyrenophora. Mycosphere, 5(2), 351–362.
Arzanlou, M., Zwiers, L. H., & Crous, P. W. (2010). Evolutionary dynamics of mating-type loci of Mycosphaerella spp. occurring on banana. Eukaryotic Cell, 9, 164–172.
Arzanlou, M., Karimi, K., & Mirabi, F. (2016). Some evidence for skewed mating type distribution in Iranian populations of Rhynchosporium commune, the cause of barley scald disease. Journal of Plant Protection Research, 56(3), 237–243.
Awasthi, L. P. (Ed.). (2015). Recent advances in the diagnosis and management of plant Diseases (p. 294). India: Springer.
Bakhshi, M., Arzanlou, M., & Babai-Ahari, A. (2011). Uneven distribution of mating type alleles in Iranian populations of Cercospora beticola, the causal agent of Cercospora leaf spot disease of sugar beet. Phytopathology Mediterranea, 50, 101–109.
Bates, J. A., & Taylor, E. J. A. (2001). Scorpion ARMS primers for SNP real-time PCR detection and quantification of Pyrenophora teres. Molecular Plant Pathology, 2(5), 275–280.
Bayraktar, H., & Akan, K. (2012). Genetic characterization of Pyrenophora graminea isolates and the reactions of some barley cultivars to leaf sripe disease under greenhouse condtions. Turkish Journal of Agriculture and Forestry, 36, 329–339.
Bogacki, P., Keiper, F. J., & Oldach, K. H. (2010). Genetic structure of South Australian Pyrenophora teres populations as revealed by microsatellite analyses. Fungal Biology, 114, 834–841.
Douhan, G. W., Peever, T. L., & Murray, T. D. (2002a). Species and mating type distribution of Tapesia yallundae and T. acuformis and occurrence of apothecia in the U.S. Pacific Northwest. Phytopathology, 92, 703–709.
Douhan, G. W., Peever, T. L., & Murray, T. D. (2002b). Multilocus population structure of Tapesia yallundae in Washington state. Molecular Ecology, 11, 2229–2239.
Elliot, C. G. (1994). Reproduction in fungi: Genetical and physiological aspects. London: Chapman&Hall.
Farr, D.F., & Rossman, A.Y. (2019). Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Retrieved March 7, 2019. Available from: https://nt.ars-grin.gov/fungaldatabases/
Ficsor, A., Bakonyi, J., Csosz, M., Tomcsanyi, A., Varga, J., & Toth, B. (2014). Occurrence of barley pathogenic Pyrenophora species and their mating types in Hungary. Cereal Research Communications, 42(4), 612–619.
Gangwar, O. P., Bhardwaj, S. C., Singh, G. P., Prasad, P., & Kumar, S. (2018). Barley disease and their management: An Indian perspective. Wheat and Barley Research, 10(3), 138–150.
Gatti, A., Rizza, F., Delogu, G., Terzi, V., Porta-Puglia, A., & Vannace, G. (1992). Physiological and biochemical variability in a population of Drechslera graminea. Journal of Genetics and Breeding, 43, 179–186.
Goodwin, S. B., Waalwijk, C., Kema, G. H., Cavaletto, J. R., & Zhang, G. (2003). Cloning and analysis of the mating-type idiomorphs from the barley pathogen Septoria passerinii. Molecular Genetics and Genomics, 269, 1–12.
Gramaje, D., Armengol, J., & Ridgway, H. J. (2013). Genetic and virulence diversity and mating type distribution of Phaeoacremonium aleophilum causing grapevine trunk diseases in Spain. European Journal of Plant Pathology, 135, 727–743.
Groenewald, M., Groenewald, J. Z., Harrington, T. C., Abeln, E. C. A., & Crous, P. W. (2006). Mating type gene analysis in apparently asexual Cercospora species is suggestive of cryptic sex. Fungal Genetics and Biology, 43, 813–825.
Groenewald, M., Linde, C. C., Groenewald, J. Z., & Crous, P. W. (2008). Indirect evidence for sexual reproduction in Cercospora beticola populations from sugar beet. Plant Pathology, 57, 25–32.
Hawksworth, D. L., Crous, P. W., Redhead, S. A., Reynolds, D. R., Samson, R. A., Seifert, K. A., Taylor, J. W., Wingfield, M. J., Abaci, Ö., Aime, C., Asan, A., Bai, F. Y., de Beer, Z. W., Begerow, D., Berikten, D., Boekhout, T., Buchanan, P. K., Burgess, T., Buzina, W., Cai, L., Cannon, P. F., Crane, J. L., Damm, U., Daniel, H. M., van Diepeningen, A. D., Druzhinina, I., Dyer, P. S., Eberhardt, U., Fell, J. W., Frisvad, J. C., Geiser, D. M., Geml, J., Glienke, C., Gräfenhan, T., Groenewald, J. Z., Groenewald, M., de Gruyter, J., Guého-Kellermann, E., Guo, L. D., Hibbett, D. S., Hong, S.-B., de Hoog, G. S., Houbraken, J., Huhndorf, S. M., Hyde, K. D., Ismail, A., Johnston, P. R., Kadaifciler, D. G., Kirk, P. M., Kõljalg, U., Kurtzman, C. P., Lagneau, P.-E., Lévesque, C. A., Liu, X., Lombard, L., Meyer, W., Miller, A., Minter, D. W., Najafzadeh, M. J., Norvell, L., Ozerskaya, S. M., Öziç, R., Pennycook, S. R., Peterson, S. W., Pettersson, O. V., Quaedvlieg, W., Robert, V. A., Ruibal, C., Schnürer, J., Schroers, H. J., Shivas, R., Slippers, B., Spierenburg, H., Takashima, M., Taskın, E., Thines, M., Thrane, U., Uztan, A. H., van Raak, M., Varga, J., Vasco, A., Verkley, G., Videira, S. I. R., de Vries, R. P., Weir, B. S., Yilmaz, N., Yurkov, A., & Zhang, N. (2011). The Amsterdam declaration on fungal nomenclature. IMA Fungus, 2, 105–112.
Jawhar, M., & Arabi, M. I. E. (2006). Genetic variability among Pyrenophora graminea isolates. Australasian Plant Pathology, 35, 279–281.
Justesen, A. F., Hansen, H. J., & Pinnschmidt, H. O. (2008). Quantification of Pyrenophora graminea in barley seed using real-time PCR. European Journal of Plant Pathology, 122, 253–263.
Lau, H. Y., & Botella, J. R. (2017). Advanced DNA-based point-of-care diagnostic methods for plant diseases detection. Frontiers in Plant Science, 8, 2016.
Leisova, L., Kucera, L., Minarikova, V., & Ovesna, J. (2005). AFLP-based PCR markers that differentiate spot and net forms of Pyrenophora teres. Plant Pathology, 54, 66–73.
Linde, C. C., & Selmes, H. (2012). Genetic diversity and mating type distribution of Tuber melanosporum and their significance to truffle cultivation in artificially planted truffieres in Australia. Applied and Environmental Microbiology, 78, 6534–6539.
Linde, C. C., Zhan, J., & McDonald, B. A. (2002). Population structure of Mycosphaerella graminicola: From lesions to continents. Phytopathology, 92, 946–955.
Linde, C. C., Zala, M., Ceccarelli, S., & McDonald, B. A. (2003). Further evidence for sexual reproduction in Rhynchosporium secalis based on distribution and frequency of mating-type alleles. Fungal Genetics and Biology, 40, 115–125.
Liu, Y. C., Double, M. L., MacDonald, W. L., Cortesi, P., & Migroom, M. G. (1996). Diversity and multilocus genetic structure in populations of Criphonectria parasitica. Phytopathology, 86, 1344–1351.
Liu, D., Coloe, S., Baird, R., & Pederson, J. (2000). Rapid mini-preparation of fungal DNA for PCR. Journal of Clinical Microbiology, 38, 471.
Marra, R. E., & Milgroom, M. G. (2001). The mating system of the fungus Cryphonectria parasitica: Selfing and self-incompatibility. Heredity, 86, 134–143.
McDonald, B. A., Zhan, J., & Burdon, J. J. (1999). Genetic structure of Rhynchosporium secalis in Australia. Phytopathology, 89, 639–645.
Milgroom, M. (1996). Recombination and multilocus structure of fungal population. Annual Review of Phytopathology, 34, 457–477.
Narmani, A., Arzanlou, M., & Babai-Ahari, A. (2015). Uneven distribution of mating-type alleles among Togninia minima isolates, one of the causal agents of leaf stripe disease on grapevines in Northwest Iran. Journal of Phytopathology, 164, 441–447.
Newton, A. C., Flavell, A. J., George, T. S., Leat, P., Mullholland, B., Ramsay, L., Revoredo-Giha, C., Russell, J., Steffenson, B. J., & Swanston, J. S. (2011). Crops that feed the world 4. Barley: A resilient crop Strenghs and weaknesses in the context of food security. Food Security, 3, 141–178.
Norvell, L. L. (2011). Report of the nomenclature committee for fungi: 17. Taxon, 60, 610–613.
Pascoe, I. G., Edwards, J., Cunnington, J. H., & Cottral, E. (2004). Detection of the Togninia teleomorph of Phaeoacremonium aleophilum in Australia. Phytopathology Mediterranea, 43, 51–58.
Ramirez-Camejo, L. A., Zuluaga-Montero, A., Lázaro-Escudero, M., Hernández-Kendall, V., & Bayman, P. (2012). Phylogeography of the cosmopolitan fungus Aspergillus flavus: Is everything everywhere? Fungal Biology, 116, 452–463.
Rau, D., Maier, F. J., Papa, R., Brown, A. H. D., Balmas, V., Saba, E., Schaefer, W., & Attene, G. (2005). Isolation and characterization of the mating-type locus of the barley pathogen Pyrenophora teres and frequencies of mating-type idiomorphs within and among fungal populations collected from barley landraces. Genome, 48, 855–869.
Rau, D., Attene, G., Brown, A. H. D., Nanni, L., Maier, F. J., Balmas, V., Saba, E., Schafer, W., & Papa, R. (2007). Phylogeny and evolution of mating-type genes from Pyrenophora teres, the causal agent of barley net blotch disease. Current Genetics, 51, 377–392.
Rooney-Latham, S., Eskalen, A., & Gubler, W. D. (2005). Teleomorph formation of Phaeoacremonium aleophilum, cause of esca and grapevine decline in California. Plant Disease, 89, 177–184.
Samanta, S., Dhua, U., Nayak, S., Behera, L., & Mukherjee, A. K. (2014). Mating types analysis of Magnaporthe oryzae populations by molecular methods. The Open Biotechnology Journal., 8, 6–12.
Sivanesan, A. (1987). Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycological Papers, 158, 180–181.
Stevens, E. A., Blakemore, E. J. A., & Reeves, J. C. (1998). Relationships amongst isolates of Pyrenophora spp. Based on the sequences of the ribosomal DNA spacer regions. Molecular Plant Pathology. Available from http://www.bspp.org.uk/mppol/1998/1111stevens.
Taylor, J. W., Jacobson, D. J., & Fisher, M. C. (1999). The evolution of asexual fungi: Reproduction, speciation and classification. Annual Review of Phytopathology, 37, 197–246.
Taylor, E. J. A., Stevens, E. A., Bates, J. A., Morreale, G., Lee, D., Kenyon, D. M., & Thomas, J. E. (2001). Rapid-cycle PCR detection of Pyrenophora graminea from barley seed. Plant Pathology, 50, 347–355.
Taylor, E. J. A., Konstantinova, P., Leigh, F., Bates, J. A., & Lee, D. (2004). Gypsy-like retrotransposone in Pyrenophora: An abundant and informative class of molecular markers. Genome., 47, 519–525.
Turgeon, B. G., & Yoder, O. C. (2000). Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genetics and Biology, 31, 1–5.
Waalwijk, C., Mendes, O., Verstappen, E. C. P., de Waard, M. A., & Kema, G. H. J. (2002). Isolation and characterization of the mating-type idiomorphs from the wheat Septoria leaf blotch fungus Mycosphaerella graminicola. Fungal Genetics and Biology, 35, 277–286.
Walters, D. R., Avrova, A., Bingham, I. J., Burnett, F. J., Fountaine, J., Havis, N. D., Hoad, S. P., Hughes, G., Looseley, M., Oxley, S. J. P., Renwick, A., Topp, C. F. E., & Newton, A. C. (2012). Control of foliar diseases in barley: Towards an integrated approach. European Journal of Plant Pathology, 133, 33–73.
Weltz, H. G., & Leonard, K. J. (1993). Phenotypic variation and parasitic fitness of races of Cochliobolus carbonum on corn in North Carolina. Phytopathology., 83, 593–601.
Wijayawardene, N. N., Hyde, K. D., Wanasinghe, D. N., Papizadeh, M., Goonasekara, I. D., Camporesi, E., Bhat, D. J., McKenzie, E. H. C., Phillips, A. J. L., Diederich, P., Tanaka, K., Li, W. J., Tangthirasunun, N., Phookamsak, R., Dai, D. Q., Dissanayake, A. J., Weerakoon, G., Maharachchikumbura, S. S. N., Hashimoto, A., Matsumura, M., Bahkali, A. H., & Wang, Y. (2016). Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Diversity, 77, 1–316.
Wingfield, M. J., DeBeer, Z. W., Slippers, B., Wingfield, B. D., Groenewald, J. Z., Lombard, L., & Crous, P. W. (2012). One fungus, one name promotes progressive plant pathology. Molecular Plant Pathology, 13, 604–613.
Xu, J. (2006). Fundamentals of fungal molecular population genetic analyses. Current Issues in Molecular Biology, 8, 75–89.
Zein, I., Jawhar, M., & Arabi, M. I. E. (2010). Efficiency of IRAP and ITS-RFLP marker systems in accessing genetic variation of Pyrenophora graminea. Genetics and Molecular Biology, 33(2), 328–332.
Zhan, J., Keema, G. H. J., Waalwijk, C., & McDonald, B. A. (2002). Distribution of mating type alleles in the wheat pathogen Mycosphaerella graminicola over spatial scales from lesions to continents. Fungal Genetics and Biology, 36, 128–136.
Zhong, S., & Steffenson, B. J. (2001). Genetic and molecular characterization of mating type genes in Cochliobolus sativus. Mycologia, 93, 852–863.
Acknowledgements
The Research Deputy of the University of Tabriz is kindly acknowledged. The authors also thank Dr. Farnaz Abed Ashtiani for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animals rights
No human and/or animal participants were involved in this research.
Informed consent
All authors consent to this submission.
Rights and permissions
About this article
Cite this article
Dokhanchi, H., Babai-Ahari, A. & Arzanlou, M. Distribution of mating type alleles in Iranian populations of Pyrenophora graminea, the causal agent of barley leaf stripe disease, using a multiplex PCR approach. Eur J Plant Pathol 156, 343–354 (2020). https://doi.org/10.1007/s10658-019-01866-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01866-0