Abstract
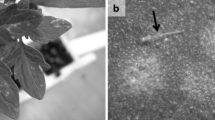
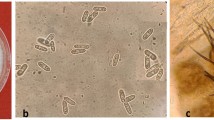
Wilting of sweet pepper plants is leading to significant yield losses in organic cultivation in glasshouses in the Netherlands. Verticillium dahliae was consistently isolated from affected plants and is known to cause wilting of plants. While sampling sweet pepper plants, root discoloration and damage of the root cortex were observed. Colletotrichum coccodes was isolated from affected roots. To study the co-occurrence and interaction of both pathogens, sweet pepper plants with, and without wilting symptoms were collected from the glasshouse. V. dahliae was only isolated from plants with wilting symptoms, while C. coccodes was also found on symptomless plants. Single or combined inoculations with V. dahliae and C. coccodes were performed on pepper seedlings to study the pathogenicity and the interaction of both pathogens. Symptom development was evaluated and fungal colonization was measured in the roots and stem with real-time PCR. V. dahliae induced stunted growth, while C. coccodes did not induce symptoms on the shoot. C. coccodes reduced root weight when plants grew under suboptimal conditions but under optimal conditions for plant growth, C. coccodes reduced V. dahliae colonization and symptom development. In conclusion, V. dahliae is the causal agent of wilting of pepper plants and C. coccodes is a weak pathogen, with antagonistic or neutral effects on symptom development and colonization by V. dahliae. This work can contribute to the understanding of soilborne diseases and their interaction with each other.






Similar content being viewed by others
References
Banno, S., Saito, H., Sakai, H., Urushiba, T., Ikeda, K., Kabe, T., et al. (2011). Quantitative nested real-time PCR detection of Verticillium longisporum and V. dahliae in the soil of cabbage fields. Journal of General Plant Pathology, 77, 282–291.
Barkdoll, A. W., & Davis, J. R. (1992). Distribution of Colletotrichum coccodes in Idaho and variation in pathogenicity on potato. Plant Disease, 76, 131–135.
Bhat, R. G., & Subbarao, K. V. (1999). Host range specificity in Verticillium dahliae. Phytopathology, 89, 1218–1225.
Buddie, A. G., Martinez-Culebras, P., Bridge, P. D., Garcia, M. D., Querol, A., Cannon, P. F., et al. (1999). Molecular characterization of Colletotrichum strains derived from strawberry. Mycological Research, 103, 385–394.
Chesters, C. G. C., & Hornby, D. (1965). Studies on Colletotrichum coccodes. Alternative host tests and tomato fruit inoculation using a typical tomato root isolate. Transactions of the British Mycological Society, 48, 583–594.
Colla, P., Gilardi, G., & Gullino, M. L. (2012). A review and critical analysis of the European situation of soilborne disease management in the vegetable sector. Phytoparasitica, 40, 515–523.
Cullen, D. W., Lees, A. K., Toth, I. K., & Duncan, J. M. (2002). Detection of Colletotrichum coccodes from soil and potato tubers by conventional and quantitative real-time PCR. Plant Pathology, 51, 281–292.
De Silva, D. D., Crous, P. W., Ades, P. K., Hyde, K. D., & Taylor, P. W. J. (2017). Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biology Reviews, 31, 155–168.
Dillard, H. R., & Cobb, A. C. (1998). Survival of Colletotrichum coccodes in infested tomato tissue and in soil. Plant Disease, 82, 235–238.
Fradin, E. F., & Thomma, B. P. H. J. (2006). Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Molecular Plant Pathology, 7, 71–86.
Garibaldi, A., Baudino, M., Minuto, A., & Gullino, M. L. (2008). Effectiveness of fumigants and grafting against tomato brown root rot caused by Colletotrichum coccodes. Phytoparasitica, 36, 483–488.
Garibaldi, A., Gilardi, G., Baudino, M., & Ortu, G. (2012). Colletotrichum coccodes : a soil-borne pathogen dangerous to a pepper rootstock in Italy. Journal of Plant Pathology, 94, S4.91.
Garmendia, I., Goicoechea, N., & Aguirreolea, J. (2004). Plant phenology influences the effect of mycorrhizal fungi on the development of Verticillium-induced wilt in pepper. European Journal of Plant Pathology, 110, 227–238.
Geboloğlu, N., Yanar, Y., Yanar, D., Akyazı, F., & Çakmak, P. (2011). Role of different rootstocks on yield and resistance for Fusarium oxysporum, Verticillium dahliae and Meloidogyne incognita in grafted peppers. European Journal of Horticultural Science, 76, 41–44.
Gilardi, G., Demarchi, S., Martano, G., Gullino, M. L., & Garibaldi, A. (2014a). Success and failures of grafting pepper against soil-borne pathogens. Acta Horticulturae, 1044, 67–72.
Gilardi, G., Colla, P., Pugliese, M., Baudino, M., Gullino, M. L., & Garibaldi, A. (2014b). Control of Colletotrichum coccodes on tomato by grafting and soil amendments. Journal of Phytopathology, 162, 116–123.
Götz, M., Nirenberg, H., Krause, S., Wolterts, H., Draeger, S., Buchner, A., et al. (2006). Fungal endophytes in potato roots studied by traditional isolation and cultivation-independent DNA-based methods. FEMS Microbiology Ecology, 58, 404–413.
Harris, C. D., Yang, J. R., & Ridout, M. S. (1993). The detection and estimation of Verticillium dahliae in naturally infested soil. Plant Pathology, 42, 238–250.
Inderbitzin, P., Davis, R. M., Bostock, R. M., & Subbarao, K. V. (2013). Identification and differentiation of Verticillium species and V. longisporum lineages by simplex and multiplex PCR assays. PloS ONE, 8, e65990.
Johnson, D. A., Geary, B., & Tsror, L. L. (2018). Potato black dot – the elusive pathogen, disease development and management. American Journal of Potato Research, 95, 340–350.
Kirkland, M.L. (1982). The roles of Verticillium dahliae, Colletotrichum atramentarium, Erwinia carotovora subsp. carotovora and E. carotovora subsp. atrospetica in “Early Dying” disease of potatoes. MSc thesis, Oregan State University.
Kotcon, J. B., Rouse, D. I., & Mitchell, J. E. (1985). Interactions of Verticillium dahliae, Colletotrichum coccodes, Rhizoctonia solani, Pratylenchus penetrans in the early dying syndrome of russet Burbank potatoes. Phytopathology, 75, 68–74.
Lees, A. K., & Hilton, A. J. (2003). Black dot (Colletotrichum coccodes): an increasingly important disease of potato. Plant Pathology, 52, 3–12.
Liu, F., Hyde, K. D., & Cai, L. (2011). Neotypification of Colletotrichum coccodes, the causal agent of potato black dot disease and tomato anthracnose. Mycology, 2, 248–254.
Liu, F., Cai, L., Crous, P. W., & Damm, U. (2013). Circumscription of the anthracnose pathogens Colletotrichum lindemuthianum and C. nigrum. Mycologia, 105, 884–860.
Livak, K. J., Schmittgen, T. D. (2001) Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25 (4):402–408
Lu, H., Zou, W. X., Meng, J. C., Hu, J., & Tan, R. X. (2000). New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Science, 151, 67–73.
Malcolm, G. M., Kuldau, G. A., Gugino, B. K., & del M Jiménez-Gasco, M. (2013). Hidden host plant associations of soilborne fungal pathogens: an ecological perspective. Phytopathology, 103, 538–544.
Michel V., & Terrettaz C. (2011). La pourriture racinaire de la tomate, causée par Colletotrichum coccodes. Factsheet, 4 pages, published on https://ira.agroscope.ch/fr-CH/publication/27192.
Nitzan, N., Hazanovsky, M., Tal, M., & Tsror, L. L. (2002). Vegetative compatibility groups in Colletotrichum coccodes, the causal agent of black dot on potato. Phytopathology, 92, 827–832.
Nitzan, N., Evans, M., & Johnson, D. A. (2006a). Colonization of potato plants after aerial infection by Colletotrichum coccodes, causal agent of potato black dot. Plant Disease, 90, 999–1003.
Nitzan, N., Lucas, B. S., & Christ, B. J. (2006b). Colonization of rotation crops and weeds by the potato black dot pathogen Colletotrichum coccodes. American Journal of Potato Research, 83, 503–507.
Nitzan, N., Cummings, T. F., & Johnson, D. A. (2008). Disease potential of soil- and tuberborne inocula of Colletotrichum coccodes and black dot severity on potato. Plant Disease, 92, 1497–1502.
Novo, M., Silvar, C., Merino, F., Martínez-Cortés, T., Lu, F., Ralph, J., et al. (2017). Deciphering the role of the phenylpropanoid metabolism in the tolerance of Capsicum annuum L. to Verticillium dahliae Kleb. Plant Science, 258, 12–20.
Pasche, J. S., Taylor, R. J., & Gudmestad, N. C. (2010). Colonization of potato by Colletotrichum coccodes: Effect of soil infestation and seed tuber and foliar inoculation. Plant Disease, 94, 905–914.
Pegg, G. F., & Brady, B. L. (2002). Verticillium wilts. Wallingford, Oxon, UK: CABI Publishing.
Redman, R. S., Dunigan, D. D., & Rodriguez, R. J. (2001). Fungal symbiosis from mutualism to parasitism: Who controls the outcome, host or invader? New Phytologist, 151, 705–716.
Schena, L., Abdelfattah, A., Mosca, S., Li Destri Nicosia, M. G., Agosteo, G. E., & Cacciola, S. O. (2017). Quantitative detection of Colletotrichum godetiae and C. acutatum sensu stricto in the phyllosphere and carposphere of olive during four phenological phases. European Journal of Plant Pathology, 149, 337–347.
Schnathorst, W. C. (1981). Life cycle and epidemiology of Verticillium. In M. E. Mace, A. A. Bell, & C. H. Beckman (Eds.), Fungal wilt diseases of plants (pp. 81–111). New York: Academic Press Inc..
Schneider, R. W., Grogan, R. G., & Kimble, K. A. (1978). Colletotrichum root-rot of greenhouse tomatoes in California. Plant Disease Reporter, 62, 969–971.
Scholte, K., Veenbaas-Rijks, J. W., & Labruyére, R. E. (1985). Potato growing in short rotations and the effect of Streptomyces spp., Colletotrichum coccodes, Fusarium tabacinum and Verticillium dahliae on plant growth an tuber yield. Potato Research, 28, 331–348.
Sedegui, M., Carroll, R. B., Morehart, A. L., & Whittington, D. P. (2000). Etiology of potato early dying in Delaware. American Journal of Potato Research, 77, 289–294.
Seo, H.-H., Park, A. R., Lee, H.-H., Park, S., Han, Y.-J., Hoang, Q. T. N., et al. (2018). A fungus-inducible pepper carboxylesterase exhibits antifungal activity by decomposing the outer layer of fungal cell walls. Molecular Plant-Microbe Interactions, 31, 505–515.
Slusarski, C., & Spotti, C. A. (2016). Efficacy of chloropicrin application by drip irrigation in controlling the soil-borne diseases of greenhouse pepper on commercial farms in Poland. Crop Protection, 89, 216–222.
Stoyanova, Z. B., Rodeva, R. M., Karov, I., Kovacevik, B., Manova, V. I., & Georgieva, R. G. (2013). Morphological and molecular characterization of Colletotrichum coccodes isolated from pepper cultivated in Bulgaria and Macedonia. Zbornik Matice srpske za prirodne nauke / Matica Srpska Proceedings for Natural Sciences, 28, 249–261.
Sukno, S. A., García, V. M., Shaw, B. D., & Thon, M. R. (2008). Root infection and systemic colonization of maize by Colletotrichum graminicola. Applied and Environmental Microbiology, 74, 823–832.
Tjamos, E. C. (1981). Virulence of Verticillium dahliae and V. albo-atrum isolates in tomato seedlings in relation to their host of origin and the applied cropping system. Phytopathology, 71, 98.
Tsror, L. L., & Hazanovsky, M. (2001). Effect of coinoculation by Verticillium dahliae and Colletotrichum coccodes on disease symptoms and fungal colonization in four potato cultivars. Plant Pathology, 50, 483–488.
Tsror, L. L., Erlich, O., Amitai, S., & Hazanovsky, M. (1998). Verticillium wilt of paprika caused by a highly virulent isolate of Verticillium dahliae. Plant Disease, 82, 437–439.
Van Hemelrijck, W., Debode, J., Heungens, K., Maes, M., & Creemers, P. (2010). Phenotypic and genetic characterization of Colletotrichum isolates from Belgian strawberry fields. Plant Pathology, 59, 853–861.
Vargas, W. A., Martin, J. M. S., Rech, G. E., Rivera, L. P., Benito, E. P., Diaz-Minguez, J. M., et al. (2012). Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotricum graminicola in Maize. Plant Physiology, 158, 1342–1358.
White, T. J., Bruns, T. D., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal genes for phylogenetics. In M. A. Innis, D. H. Gelfrand, J. J. Sninsky, & T. J. White (Eds.), PCR Protocols (pp. 315–322). San Diego: Academic Press.
Wicklow, D. T., Jordan, A. M., & Gloer, J. B. (2009). Antifungal metabolites (monorden, monocillins I, II, III) from Colletotrichum graminicola, a systemic vascular pathogen of maize. Mycological Research, 113, 1433–1442.
Zou, W. X., Meng, J. C., Lu, H., Chen, G. X., Shi, G. X., Zhang, T. Y., et al. (2000). Metabolites of Colletotrichum gloeosporioides, an endophytic fungus in Artemisia mongolica. Journal of Natural Products, 63, 1529–1530.
Acknowledgements
The authors would like to thank the farmer Frank de Koning for his hospitality, Ilse Delaere and Nadia Lemeire for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
Authors declare that they have no conflict of interest (financial or non-financial).
Research involving human participants and/or animals
This research does not involve human participants and/or animals.
Informed consent
Not necessary, the research does not involve human participants.
Electronic supplementary material
Fig. S1
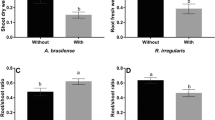
Plant height after inoculation of V. dahliae and/or C. coccodes. Data are pooled from repetition 1 (24 dpi) and repetition 2 (27 dpi). 3-week old seedlings were inoculated by the root-dip method. Control: water; Vd: 103 V. dahliae mL−1; Cc(3) and Cc(4): 103 or 2.7 × 104 conidia of C. coccodes mL−1; Vd + Cc(3) and Vd + Cc(4): 103 conidia of V. dahliae mL−1 + 103 or 2.7 × 104 conidia of C. coccodes mL−1. Data are presented as boxplots from 18 plants. Different letters indicate results, which differ significantly at p < 0.05 by the Dunn Test. (PDF 88 kb)
Fig. S2
Plant height (a) and relative colonization by C. coccodes of the roots (b) and V. dahliae of the roots (c) and stem (d) of pepper plants inoculated with V. dahliae and/or C. coccodes, 41 dpi. 3-week old seedlings were inoculated by the root-dip method. Control: water; Vd(4): 104 V. dahliae mL−1; Cc(3) and Cc(4): 1 × 103 or 2.7 × 104 conidia of C. coccodes mL−1; Vd(4) + Cc(3) and Vd(4) + Cc(4): 104 conidia of V. dahliae mL−1 + 103 or 2.7 × 104 conidia of C. coccodes mL−1. For the plant height, data are presented as boxplots from 6 plants. Different letters indicate results, which differ significantly at p < 0.05 by the Dunn Test (a). For relative colonization, six plants per treatment were analyzed and the number of plants in which C. coccodes or V. dahliae could be detected is indicated above the bars (n). Data are presented as boxplots from the n plants.* only five plants were analyzed instead of six (b,c,d). (PDF 267 kb)
Rights and permissions
About this article
Cite this article
Tyvaert, L., Everaert, E., Lippens, L. et al. Interaction of Colletotrichum coccodes and Verticillium dahliae in pepper plants. Eur J Plant Pathol 155, 1303–1317 (2019). https://doi.org/10.1007/s10658-019-01857-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01857-1