Abstract
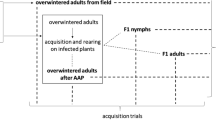
Ricania speculum is an Asian planthopper that was accidentally imported into Europe from the Far East. The planthopper was first observed in Italy in 2009 (Genoa province, Liguria) and is now considered established. The current range of R. speculum in Italy includes Liguria, Tuscany, Veneto, Piedmont and Latium. Ricania speculum is polyphagous and has been observed on wild and cultivated plants that are herbaceous or woody. This phloem feeder frequently feeds on Vitis spp. (cultivated and wild grapevines) and Clematis vitalba plants that are hosts of Flavescence dorée phytoplasma (FDp), a quarantine phloem-limited bacterial pathogen and a major threat to viticulture of several European regions. The aim of this work was to assess if R. speculum could act as a vector of FDp, thereby affecting disease epidemiology. To explore the role of R. speculum in FDp transmission, nymphs were allowed to feed on FDp-infected Vicia faba plants to estimate acquisition efficiency and successively transferred onto Vitis vinifera, V. faba and C. vitalba test plants to determine transmission ability. Ricania speculum was unable to transmit FDp; some individuals acquired the phytoplasma but supported a very low level of pathogen multiplication, compared with the competent vector. Consistent with transmission results, FDp was detected only in one out of 51 tested salivary gland samples. According to our results, the role of R. speculum in FD epidemiology is expected to be negligible.


Similar content being viewed by others
References
Arricau-Bouvery, N., Duret, S., Dubrana, M.-P., Batailler, B., Desqué, D., Béven, L., et al. (2018). Variable membrane protein a of flavescence dorée phytoplasma binds the midgut perimicrovillar membrane of Euscelidius variegatus and promotes adhesion to its epithelial cells. Applied and Environmental Microbiology, 84, 8–17.
Bosco, D., & D'Amelio, R. (2010). Transmission specificity and competition of multiple phytoplasmas in the insect vector. In P. G. Weintraub & P. Jones (Eds.), Phytoplasmas: Genomes, plant hosts and vectors (pp. 293–308). Wallingford: CAB International.
Bressan, A., Clair, D., Sémétey, O., & Boudon-Padieu, E. (2006). Insect injection and artificial feeding bioassays to test the vector specificity of Flavescence dorée phytoplasma. Phytopathology, 96(7), 790–796.
Caudwell, A., Kuszala, C., Larrue, J., & Bachelier, J. (1972). Transmission de la Flavescence dorée de la fève à la fève par des cicadelles des genres Euscelis et Euscelidius. Ann Phytopathol, No. hors série, 181–189.
Chuche, C., & Thiery, D. (2014). Biology and ecology of the Flavescence dorée vector Scaphoideus titanus: A review. Agronomy for Sustainable Development, 34, 381–403.
EFSA Panel on Plant Health (PLH), Jeger, M., Bragard, C., Caffier, D., Candresse, T., Chatzivassiliou, E., et al. (2016). Risk to plant health of Flavescence dorée for the EU territory. EFSA Journal, 14(12), 4603.
Eveillard, S., Jollard, C., Labroussaa, F., Khalil, D., Perrin, M., Desqué, D., et al. (2016). Contrasting susceptibilities to Flavescence dorée in Vitis vinifera, rootstocks and wild Vitis species. Frontiers in Plant Science, 7, 1762.
Filippin, L., Jović, J., Cvrković, T., Forte, V., Clair, D., Toševski, I., Boudon-Padieu, E., Borgo, M., & Angelini, E. (2009). Molecular characteristics of phytoplasmas associated with Flavescence dorée in clematis and grapevine and preliminary results on the role of Dictyophara europaea as a vector. Plant Pathology, 58(5), 826–837.
Galetto, L., Nardi, M., Saracco, P., Bressan, A., Marzachì, C., & Bosco, D. (2009). Variation in vector competency depends on chrysanthemum yellows phytoplasma distribution within Euscelidius variegatus. Entomologia Experimentalis et Applicata, 131(2), 200–207.
Galetto, L., Marzachì, C., Demichelis, S., & Bosco, D. (2011a). Host plant determines the phytoplasma transmission competence of Empoasca decipiens (Hemiptera: Cicadellidae). Journal of Economic Entomology, 104, 360–366.
Galetto, L., Bosco, D., Balestrini, R., Genre, A., Fletcher, J., & Marzachì, C. (2011b). The major antigenic membrane protein of ‘Candidatus Phytoplasma asteris’ selectively interacts with ATP synthase and actin of leafhopper vectors. PLoS One, 6(7), e22571.
Germain, J. F., Rossi, E., & Lucchi, A. (2016). Ricania speculum, un nouvel hémiptère en Méditerranée. Phytoma, 697, 7–9.
Hogenhout, S. A., Oshima, K., Ammar, E.-D., Kakizawa, S., Kingdom, H. N., & Namba, S. (2008). Phytoplasmas: Bacteria that manipulate plants and insects. Molecular Plant Pathology, 9(4), 403–423.
Lee, I.-M., Hammond, R. W., Davis, R. E., & Gundersen, D. E. (1993). Universal amplification and analysis of pathogen 16S rDNA for classification and identification of mycoplasmalike organisms. Phytopathology, 83(8), 834–842.
Lessio, F., Picciau, L., Gonella, E., Mandrioli, M., Tota, F., & Alma, A. (2016). The mosaic leafhopper Orientus ishidae: Host plants, spatial distribution, infectivity, and transmission of 16SrV phytoplasmas to vines. Bulletin of Insectology, 69(2), 277–289.
Lu, H., Wilson, B. A. L., Ash, G. J., Woruba, S. B., Fletcher, M. J., You, M., Yang, G., & Gurr, G. M. (2016). Determining putative vectors of the Bogia coconut syndrome phytoplasma using loop-mediated isothermal amplification of single-insect feeding media. Scientific Reports, 6(1), 35801.
Maixner, M., Reinert, W., & Darimont, H. (2000). Transmission of grapevine yellows by Oncopsis alni (Schrank) (Auchenorrhyncha: Macropsinae). Vitis, 39, 83–84.
Marzachí, C., & Bosco, D. (2005). Relative quantification of chrysanthemum yellows (16Sr I) phytoplasma in its plant and insect host using real-time polymerase chain reaction. Molecular Biotechnology, 30(2), 117–128.
Mazza, G., Pennacchio, F., Gargani, E., Franceschini, I., Roversi, P. I., & Cianferoni, F. (2014). First report of Ricania speculum (Walker, 1851) in Europe (Hemiptera: Fulgoromorpha: Ricaniidae). Zootaxa, 3861, 297–300.
Mazza, G., Marraccini, D., Lucchi, A., Marianelli, L., Sabbatini Peverieri, G., Bosio, G., Giacometto, E., Rapa, L., Cianferoni, F., Roversi, P. F., & Gargani, E. (2018). First record of Ricania speculum (Walker, 1851) (Hemiptera: Ricaniidae) from Veneto, Piedmont and Latium regions and new host plants. Redia, 101, 197–200.
Pelletier, C., Salar, P., Gillet, J., Cloquemin, G., Very, P., Foissac, X., & Malembic-Maher, S. (2009). Triplex real-time PCR assay for sensitive and simultaneous detection of grapevine phytoplasmas of the 16SrV and 16SrXII-A groups with an endogenous analytical control. Vitis, 48(2), 87–95.
Rashidi, M., D’Amelio, R., Galetto, L., Marzachì, C., & Bosco, D. (2014). Interactive transmission of two phytoplasmas by the vector insect. Annals of Applied Biology, 165(3), 404–413.
Rashidi, M., Galetto, L., Bosco, D., Bulgarelli, A., Vallino, M., Veratti, F., & Marzachì, C. (2015). Role of the major antigenic membrane protein in phytoplasma transmission by two insect vector species. BMC Microbiology, 15(1), 193. https://doi.org/10.1186/s12866-015-0522-5.
Roggia, C., Caciagli, P., Galetto, L., Pacifico, D., Veratti, F., Bosco, D., & Marzachì, C. (2014). Flavescence dorée phytoplasma titre in field-infected Barbera and Nebbiolo grapevines. Plant Pathology, 63(1), 31–41.
Rossi, E., StroiŃski, A., & Lucchi, A. (2015). Egg morphology, laying behavior and record of the host plants of Ricania speculum (Walker, 1851), a new alien species for Europe (Hemiptera: Ricaniidae). Zootaxa, 4044(1), 93–104.
Rossi, M., Pegoraro, M., Ripamonti, M., Abbà, S., Beal, D., Giraudo, A., Veratti, F., Malembic-Maher, S., Salar, P., Bosco, D., & Marzachì, C. (2019). Genetic diversity of Flavescence dorée phytoplasmas at vineyard scale. Applied and Environmental Microbiology. https://doi.org/10.1128/AEM.03123-18.
Salar, P., Charenton, C., Foissac, X., & Malembic-Maher, S. (2013). Multiplication kinetics of Flavescence dorée phytoplasma in broad bean. Effect of phytoplasma strain and temperature. European Journal of Plant Pathology, 135(2), 371–381.
Suzuki, S., Oshima, K., Kakizawa, S., Arashida, R., Jung, H.-Y., Yamaji, Y., Nishigawa, H., Ugaki, M., & Namba, S. (2006). Interaction between the membrane protein of a pathogen and insect microfilament complex determines insect-vector specificity. Proceedings of the National Academy of Sciences, 103(11), 4252–4257.
Tomkins, M., Kliot, A., Marée, A. F., & Hogenhout, S. A. (2018). A multi-layered mechanistic modelling approach to understand how effector genes extend beyond phytoplasma to modulate plant hosts, insect vectors and the environment. Current Opinion in Plant Biology, 44, 39–48.
Trivellone, V. (2019). An online global database of Hemiptera-Phytoplasma-plant biological interactions. Biodiversity Data Journal, 7, e32910.
Wilson, S. W., Rossi, E., & Lucchi, A. (2016). Descriptions of the adult genitalia and immatures of the Asian Planthopper Ricania speculum (Hemiptera: Fulgoroidea: Ricaniidae) recently introduced to Italy. Annals of the Entomological Society of America, 109(6), 899–905.
Acknowledgements
The authors would like to thank Caterina Perrone, Elena Zocca and Ivana Gribaudo for providing test plants and Flavio Veratti for routine phtoplasma strains maintaining.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: D. Bosco, C. Marzachì and A. Lucchi. Performed the experiments: M. Pegoraro, L. Galetto and E. Rossi. Analyzed the data: M. Pegoraro, L. Galetto and D. Bosco. Wrote the paper: L. Galetto. Critical revision of the manuscript: all authors.
Corresponding author
Ethics declarations
The work complies to the ethical standards of this journal.
Conflict of interest
The authors declare that they have no conflict of interest. This research did not involve human participants. The manuscript was not previously published. All authors contributed and agreed to submission to EJPP.
Rights and permissions
About this article
Cite this article
Galetto, L., Pegoraro, M., Marzachì, C. et al. Potential role of the alien planthopper Ricania speculum as vector of Flavescence dorée phytoplasma. Eur J Plant Pathol 154, 1103–1110 (2019). https://doi.org/10.1007/s10658-019-01731-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01731-0