Abstract
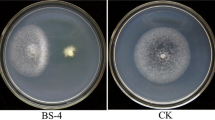
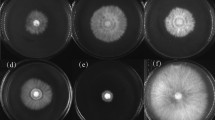
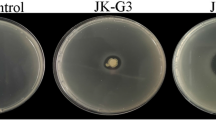
Mustard clubroot, caused by Plasmodiophora brassicae, is a serious disease that affects Brassica juncea var. tumida Tsen, a mustard plant that is the raw material for a traditional fermented food manufactured in the Chongqing Municipality, People’s Republic of China. To find antagonistic bacteria for P. brassicae, 124 bacteria were obtained from the rhizosphere soil of B. juncea var. tumida grown in Fuling, Chongqing. Isolates were preliminarily chosen by evaluating the inhibition rate of the P. brassicae resting spore germination. The biocontrol effects of three antagonistic bacteria against clubroot on B. juncea var. tumida were evaluated in a greenhouse experiment. B18 showed the highest control efficiency, at 63.4% in the greenhouse test. In a field trial, B18 was also effective in controlling clubroot, but only at a 49.7% efficiency rate. According to 16S rDNA sequence analysis, strain B18 had a 100% sequence similarity with type strain Zhihengliuella aestuarii DY66T (EU939716). Based on morphological, cultural, physiological and biochemical characteristics, the DNA G + C content, polar lipids, fatty acids, cell wall analysis, as well as DNA–DNA hybridization, strain B18 was identified as Z. aestuarii B18. Thus, the isolate B18 might have a potential biocontrol application for clubroot. We report for the first time that Z. aestuarii B18 can control clubroot.




Similar content being viewed by others
References
Arie, T., Kobayashi, Y., Okada, G., Kono, Y., & Yamaguchi, I. (1998). Control of soilborne clubroot disease of cruciferous plants by epoxydon from Phoma Glomerata. Plant Pathology, 47, 743–748.
Baik, K. S., Lim, C. H., Park, S. C., Choe, H. N., Kim, H. J., Kim, D., Lee, K. H., & Seong, C. N. (2011). Zhihengliuella aestuarii sp. nov., isolated from tidal flat sediment. International Journal of Systematic and Evolutionary Microbiology, 61(7), 1671–1676.
Cheah LH, Page BBC (1997) Trichoderma spp. For potential biocontrol of clubroot of vegetable brassicas. Proc 50th N Z Plant Protection Conf 150–153.
Cordovez, V., Carrion, V. J., Etalo, D. W., Mumm, R., Zhu, H., van Wezel, G. P., & Raaijmakers, J. M. (2015). Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Frontiers in Microbiology, 6, 1081.
De Ley, J. (1970). Reexamination of the association between melting point, buoyant density and chemical base composition of deoxyribonucleic acid. Journal of Bacteriology, 101, 738–754.
De Ley, J., Cattoir, H., & Reynaerts, A. (1970). The quantitative measurement of DNA hybridization from renaturation rates. European Journal of Biochemistry, 12, 133–142.
Dixon, G. R. (2009). Plasmodiophora brassicae In its environment. Plant Growth Regulation, 28, 212–228.
Donald, C., & Porter, I. (2009). Integrated control of clubroot. Plant Growth Regulation, 28, 289.
El-Tarabily, K. A., & Sivasithamparam, K. (2006). Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biology & Biochemistry, 38, 1505–1520.
Faffian, R., & Strelkov, S. E. (2009). Detection and measurement of Plasmodiophora brassicae. Journal of Plant Growth Regulation, 28, 282–288.
Guo, S. Y., Mao, Z. C., Wu, Y. X., Hao, K., He, P. F., & He, Y. Q. (2013). Genome sequencing of Bacillus Subtilis strain XF-1 with high efficiency in the suppression of Plasmodiophora brassicae. Genome Announcements, 1(2), 1–2.
Howell, C. R. (2003). Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Disease, 87(1), 4–10.
Jaschke, D., Dugassa-Gobena, D., Karlovsky, P., Vidal, S., & Ludwig-Muller, J. (2010). Suppression of clubroot (Plasmodiophora brassicae) development in Arabidopsis thaliana by the endophytic fungus Acremonium alternatum. Plant Pathology, 59, 100–111.
Khaled, A. E.-T., & Krishnapillai, S. (2006). Non-streptomycete actinomycetes as biocontrol agents of soil-borne fungal plant pathogens and as plant growth promoters. Soil Biology & Biochemistry, 38, 1505–1520.
Kuginuki, Y., Yoshikawa, H., & Hirai, M. (1999). Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot-resistant cultivars of Chinese cabbage (Brassica rapa L. ssp. pekinensis). Eur J Plant Pathol, 105, 327.
Lee, S. O., Choi, G. J., Choi, Y. H., Jang, K. S., Park, D. J., Kim, C. J., & Kim, J. C. (2008). Isolation and characterization of endophytic actinomycetes from Chinese cabbage roots as antagonists to Plasmodiophora brassicae. Journal of Microbiology and Biotechnology, 18(11), 1741–1746.
Ludwig-Muller, J., & Schuller, A. (2008). What can we learn from clubroots: Alterations in host roots and hormone homeostasis caused by Plasmodiophora brassicae. European Journal of Plant Pathology, 121, 291–302.
Marmur, J., & Doty, P. (1962). Determination Of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol, 5, 109–118.
Mesbah, M., Premachandran, U., & Whitman, W. B. (1989). Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid chromatography. International Journal of Systematic Bacteriology, 39, 159–167.
Michelsen, C. F., Watrous, J., Glaring, M. A., Kersten, R., Koyama, N., Dorrestein, P. C., & Stougaard, P. (2015). Nonribosomal peptides, key biocontrol components for Pseudomonas fluorescens In5, isolated from a Greenlandic suppressive soil. MBio, 6, e00079–e00015. doi:10.1128/mBio.00079-15.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497.
Naiki, T., Dixon, G. R., & Ikegami, H. (1987). Quantitative estimation of spore germination of Plasmodiophora brassicae. Transactions of the British Mycological Society, 89, 569–609.
Narisawa, K., Shimura, M., Usuki, F., Fukuhara, S., & Hashiba, T. (2005). Effects of pathogen density, soil moisture, and soil pH on biological control of clubroot in Chinese cabbage by Heteroconium chaetospira. Plant Disease, 89, 285–290.
Rashid, A., Ahmed, H. U., Xiao, Q., Hwang, S. F., & Strelkov, S. E. (2013). Effects of root exudates and pH on Plasmodiophora brassicae resting spore germination and infection of canola (Brassica napus L.) root hairs. Crop Protection, 48, 16–23.
Sasser M (1990) Identification of bacteria by gas chromatography of cellular fatty acids. Technical note 101. Newark, DE: Microbial ID.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
Wang, J., Huang, Y., Lin, S., Liu, F., Song, Q., Peng, Y. L., & Zhao, L. (2012). A strain of Streptomyces griseoruber isolated from rhizospheric soil of Chinese cabbage as antagonist to Plasmodiophora brassicae. Annales de Microbiologie, 62, 247–253.
Xiao, C., & Guo, X. (2002). Biological Charateristic of Plasmodiophora brassicae. Mycosystema, 21(4), 597–603.
Zhou, L., Li, M., Yang, J., Wei, L., & Ji, G. (2014). Draft genome sequence of antagonistic agent Lysobacter antibioticus 13-6. Genome Announcements, 2(5), e00566–e00514.
Acknowledgements
This work was financially supported by the Natural Science Foundation of Chongqing Science and Technology Commission (grant number: cstc 2013jcyjA80038) and the Special Fund for Post-Doctoral Research Project of Chongqing (grant number: Xm2015046)
Author information
Authors and Affiliations
Contributions
CYZ and YLL conceived and designed the study. YLL, DWD, ZQG, HJ, XYW, YFY and CJW collected samples and performed the experiment. YLL carried out the data analysis. YLL and CYZ contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no potential conflicts of interest.
Informed consent
All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Luo, Y., Dong, D., Gou, Z. et al. Isolation and characterization of Zhihengliuella aestuarii B18 suppressing clubroot on Brassica juncea var. tumida Tsen. Eur J Plant Pathol 150, 213–222 (2018). https://doi.org/10.1007/s10658-017-1269-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1269-7