Abstract
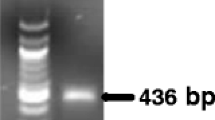
Rhizoctonia cerealis causes sharp eyespot in cereals and the pathogen survives as mycelia or sclerotia in soil. Real-time Polymerase Chain Reaction (qPCR) assays based on TaqMan chemistry are highly suitable for use on DNA extracted from soil. We report here the first qPCR assay for R. cerealis using TaqMan primers and a probe based on a unique Sequence Characterised Amplified Region (SCAR). The assay is highly specific and did not amplify DNA from a range of other binucleate Rhizoctonia species or isolates of anastomosis groups of Rhizoctonia solani. The high sensitivity of the assay was demonstrated in soils using a bulk DNA extraction method where 200 μg sclerotia in 50 g of soil were detected. DNA of the pathogen could also be amplified from asymptomatic wheat plants. Using the assay on soil samples from fields under different crop rotations, R. cerealis was most frequently detected in soils where wheat was grown or soil under pasture. It was detected least frequently in fields where potatoes were grown. This study demonstrates that assays derived from SCAR sequences can produce specific and sensitive qPCR assays.



Similar content being viewed by others
References
Alaeddini, R. (2012). Forensic implications of PCR inhibition—a review. Forensic Science International: Genetics, 6, 297–305.
Boine, B., Renner, A. C., Zellner, M., & Nechwatal, J. (2014). Quantitative methods for assessment of the impact of different crops on the inoculum density of Rhizoctonia solani AG2-2IIIB in soil. European Journal of Plant Pathology, 140, 745–756.
Broders, K. D., Parker, M. L., Melzer, M. S., & Boland, G. J. (2014). Phylogenetic diversity of Rhizoctonia solani associated with canola and wheat in Alberta, Manitoba, and Saskatchewan. Plant Disease 98(12), 1695–1701.
Brown, M.B., Woodhall, J.W., Mooney, S.J., & Ray, R.V. (2014). The occurrence and population dynamics of Rhizoctonia solani in soil of winter wheat. Proeceedings of the Dundee Conference. Crop Protection in Northern Britain 2014, Dundee, 25-26 February 2014, 107–112
Budge, G. E., Shaw, M. W., Colyer, A., Pietravalle, S., & Boonham, N. (2009). Molecular tools to investigate Rhizoctonia solani distribution in soil. Plant Pathology, 58, 1071–1080.
Chen, H. G., Fang, Z., De, H. L., Lin, L., & Wang, Y. Z. (2005). PCR based detection of Rhizoctonia cerealis. Acta Physica Sinica, 32, 261–265.
Clarkson, J. D. S., & Cook, R. J. (1983). Effect of sharp eyespot (Rhizoctonia cerealis) on yield losses in winter wheat. Plant Pathology, 32, 421–428.
Colbach, N., Lucas, P., Cavelier, N., & Cavelier, A. (1997). Influence of cropping system on sharp eyespot in winter wheat. Crop Protection, 16, 415–422.
Cubeta, M. A., & Vilgalys, R. (1997). Population biology of the Rhizoctonia solani complex. Phytopathology, 87, 480–484.
Goll, M. B., Schade-Schütze, A., Swart, G., Oostendorp, M., Schott, J. J., Jaser, B., & Felsenstein, F. G. (2014). Survey on the prevalence of Rhizoctonia spp. in European soils and determination of the baseline sensitivity towards sedaxane. Plant Pathology, 63, 148–154.
Guo, Y., Li, W., Sun, H., Wang, N., Yu, H., & Chen, H. (2012). Detection of Rhizoctonia cerealis in soil using real-time PCR. Journal of General Plant Pathology, 78, 247–254.
Hamada, M. S., Yin, Y., Chen, H., & Ma, Z. (2011). The escalating threat of Rhizoctonia cerealis, the causal agent of sharp eyespot in wheat. Pest Management Science, 67, 1411–1419.
Hamada, M. S., Yin, Y. N., & Ma, Z. H. (2012). Detection of Rhizoctonia cerealis in wheat tissues by a real-time PCR assay. Journal of Plant Pathology, 94, 215–217.
Hollins, T. W., Jellis, G. J., & Scott, P. R. (1983). Infection of potato and wheat by isolates of Rhizoctonia solani and R. cerealis. Plant Pathology, 32, 303–310.
Lees, A. K., Cullen, D. W., Sullivan, L., & Nicolson, M. J. (2002). Development of conventional and quantitative real-time PCR assays for the detection and identification of Rhizoctonia solani AG3 in potato and soil. Plant Pathology, 51, 293–302.
Lemańczyk, G. (2012). The role of the preceding crop and weed control in the transmission of Rhizoctonia cerealis and R. solani to winter cereals. Journal of Plant Protection Research, 52, 93–105.
Lemańczyk, G., & Kwaśna, H. (2013). Effects of sharp eyespot (Rhizoctonia cerealis) on yield and grain quality of winter wheat. European Journal of Plant Pathology, 135, 187–200.
Li, W., Sun, H., Deng, Y., Zhang, A., & Chen, H. (2013). The heterogeneity of the DNA ITS sequences and its phylogeny in Rhizoctonia cerealis, the cause of sharp eyespot in wheat. Current Genetics, 60, 1–9.
Nicholson, P., & Parry, D. W. (1996). Development and use of a PCR assay to detect Rhizoctonia cerealis, the cause of sharp eyespot in wheat. Plant Pathology, 45, 872–873.
Okubara, P. A., Schroeder, K. L., & Paulitz, T. C. (2008). Identification and quantification of Rhizoctonia solani and R. oryzae using real-time polymerase chain reaction. Phytopathology, 98, 837–847.
Ophel-Keller, K., Mckay, A., Hartley, D., Herdina, & Curran, J. (2008). Development of a routine DNA-based testing service for soilborne diseases in Australia. Australasian Plant Pathology, 37, 243–253.
Ray, R. V., Jenkinson, P., & Edwards, S. G. (2004). Effects of fungicides on eyespot, caused predominantly by Oculimacula acuformis, and yield of early-drilled winter wheat. Crop Protection, 23, 1199–1207.
Scott, P. R., & Hollins, T. W. (1974). Effects of eyespot on the yield of winter wheat. Annals of Applied Biology, 78, 269–279.
Sharon, M., Kuninaga, S., Hyakumachi, M., Naito, S., & Sneh, B. (2008). Classification of Rhizoctonia spp. using rDNA-ITS sequence analysis supports the genetic basis of the classical anastomosis grouping. Mycoscience, 49, 93–114.
Sneh, B., Burpee, L., & Ogoshi, A. (1991). Identification of Rhizoctonia species 133 pp. St. Paul, APS Press.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28, 2731–2739.
Woodhall, J. W., Lees, A. K., Edwards, S. G., & Jenkinson, P. (2008). Infection of potato by Rhizoctonia solani: effect of anastomosis group. Plant Pathology, 57, 897–905.
Woodhall, J. W., Webb, K. M., Giltrap, P. M., Adams, I. P., Peters, J. C., Budge, G. E., & Boonham, N. (2012a). A new large scale soil DNA extraction procedure and real-time PCR assay for the detection of Sclerotium cepivorum in soil. European Journal of Plant Pathology, 134, 467–473.
Woodhall, J. W., Laurenson, L., & Peters, J. C. (2012b). First report of Rhizoctonia solani anastomosis group 5 (AG5) in wheat in the UK. New Disease Reports, 26, 9.
Woodhall, J. W., Adams, I. P., Peters, J. C., Harper, G., & Boonham, N. (2013). A new quantitative real-time PCR assay for Rhizoctonia solani AG3-PT and the detection of AGs of Rhizoctonia solani associated with potato in soil and tuber samples in great Britain. European Journal of Plant Pathology, 136, 273–280.
Yang, S., Lin, S., Kelen, G. D., Quinn, T. C., Dick, J. D., Gaydos, C. A., & Rothman, R. E. (2002). Quantitative multiprobe PCR assay for simultaneous detection and identification to species level of bacterial pathogens. Journal of Clinical Microbiology, 40, 3449–3454.
Acknowledgments
The authors would like to acknowledge Syngenta Global for funding the PhD studentship of Matthew Brown at the University of Nottingham. Eder Somoza Valdeolmillos was supported by the EU Leonardo da Vinci programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woodhall, J.W., Brown, M.J., Perkins, K. et al. A TaqMan real-time PCR assay for Rhizoctonia cerealis and its use in wheat and soil. Eur J Plant Pathol 148, 237–245 (2017). https://doi.org/10.1007/s10658-016-1083-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-1083-7