Abstract
Cercospora leaf spot (CLS), caused by Cercospora beticola, is the most destructive foliar disease and is a problem in sugar beet production areas, such as Central High Plains (states of Colorado, Montana, Nebraska and Wyoming) in the United States. The disease can be controlled by strobilurin fungicides, referred to as quinone outside inhibitors (QoIs), with a single target site on C. beticola. Strobilurin resistance has been reported in beet production areas from the United States, including the Central High Plains. Although strobilurin resistance is quantitatively inherited, it is considered that it has low to medium heritability in the population. Effective diagnostic tools are required for the rapid detection of C. beticola strobilurin resistance. The study obtained a partial nucleotide sequence of the C. beticola cytochrome b gene and determined to a putative protein with ~386 amino acid residues. Eighty C. beticola isolates (2004–2011) from the Central High Plains were analyzed for mutations. We found a single nucleotide polymorphic (SNP) site which led to G143A mutation and was present in 2 C. beticola QoI-resistant isolates. Partial sequences obtained from 82 C. beticola QoI-sensitive isolates showed identical cytochrome b gene. We developed a PCR-RFLP assay that involved an in vitro digestion using Fnu4HI restriction enzyme for the rapid molecular detection of G143A mutation in the C. beticola population. Results indicated the PCR-RFLP assay was reliable, sensitive, and can be used for the rapid detection of C. beticola strobilurin resistance.


Similar content being viewed by others
References
Bartlett, D. W., Clough, J. M., Godwin, J. R., Hall, A. A., Hamer, M., & Parr-Dobrzanski, B. (2002). The strobilurin fungicides. Pest Management Science, 58, 649–662.
Bäumler, S., Felsenstein, F. G., & Schwarz, G. (2003). CAPS and DHPLC analysis of a single nucleotide polymorphism in the cytochrome b gene conferring resistance to strobilurins in field isolates of Blumeria graminis f. sp. hordei. Journal of Phytopathology, 151, 149–152.
Birla, K., Rivera-Varas, V., Secor, G., Khan, M., & Bolton, M. (2012). Characterization of cytochrome b from European field isolates of Cercospora beticola with quinone outside inhibitor resistance. European Journal of Plant Pathology, 134, 475–488.
Blanco, E., Parra, G., & Guigó, R. (2007). Using geneid to identify genes. Current Protocols in Bioinformatics, 18, 4.3.1–4.3.28.
Bolton, M. D., Rivera, V., & Secor, G. (2012). Identification of the G143A mutation associated with QoI resistance in Cercospora beticola field isolates from Michigan, United States. Pest Management Science, 69, 35–39.
Brent, K. J., & Hollomon, D. W. (2007). Fungicide resistance: the assessment of risk. FRAC monograph no 2, 2nd Edn (pp.1–53). Brussels: Global Crop Protection Federation.
Briere, S. C., Franc, G. D., & Kerr, E. D. (2001). Fungicide sensitivity characteristics of Cercospora beticola isolates recovered from the High Plains of Colorado, Montana, Nebraska, and Wyoming. 1. Benzimidazole and triphenyltin hydroxide. Journal Sugar Beet Research, 38, 111–120.
Briere, S. C., Franc, G. D., & Kerr, E. D. (2003). Fungicide sensitivity characteristics of Cercospora beticola isolates recovered from the high plains region of Colorado, Montana, Nebraska, and Wyoming. 2. Mancozeb, propiconazole, and axozystrobin. Journal Sugar Beet Research, 14, 1–2.
Brown, J. K. M., & Wolfe, M. S. (1990). Structure and evolution of a population of Erysiphe graminis f. sp. hordei. Plant Pathology, 39, 376–390.
Bugbee, W. M. (1995). Cercospora beticola tolerant to triphenyltin hydroxide. Journal Sugar Beet Research, 32, 167–174.
Calpouzos, L., & Stallknecht, G. F. (1966). Photoperiodism by conidiophores of Cercospora beticola. Phytopathology, 56, 702–704.
Cape, J. L., Bowman, M. K., & Kramer, D. M. (2006). Understanding the cytochrome bc complexes by what they don’t do. The Q-cycle at 30. Trends in Plant Science, 11, 46–55.
Crofts, A. R. (2004). The cytochrome bc1 complex: function in the context of structure. Annual Review of Physiology, 66, 689–733.
Crofts, A. R., Hong, S., Ugulava, N., Barquera, B., Gennis, R., Guergova-Kuras, M., et al. (1999). Pathways for proton release during ubihydroquinone oxidation by the bc1 complex. Proceedings of the National Academy of Sciences of the United States of America, 96, 10021–10026.
Darrouzet, E., Moser, C. C., Dutton, P. L., & Daldal, F. (2001). Large scale domain movement in cytochrome bc 1: a new device for electron transfer in proteins. Trends in Biochemical Sciences, 26, 445–451.
Di Rago, J. P., Coppee, J. Y., & Colson, A. M. (1989). Molecular basis for resistance to myxothiazol, mucidin (strobilurin a), and stigmatellin. Cytochrome b inhibitors acting at the center o of the mitochondrial ubiquinol-cytochrome c reductase in Saccharomyces cerevisiae. Journal of Biological Chemistry, 264, 14543–14548.
Duffus, J. E., & Ruppel, E. G. (1993). Diseases. In D. A. Cooke & R. K. Scott (Eds.), The sugar beet crop: science into practice (pp. 346–427). London: Chapman & Hall.
Esposti, M. D., De Vries, S., Crimi, M., Ghelli, A., Patarnello, T., & Meyer, A. (1993). Mitochondrial cytochrome b: evolution and structure of the protein. BBA-Bioenergetics, 1143, 243–271.
Gisi, U., Chin, K. M., Knapova, G., Küng Färber, R., Mohr, U., Parisi, S., et al. (2000). Recent developments in elucidating modes of resistance to phenylamide, DMI and strobilurin fungicides. Crop Protection, 19, 863–872.
Gisi, U., Sierotzki, H., Cook, A., & McCaffery, A. (2002). Mechanisms influencing the evolution of resistance to Qo inhibitor fungicides. Pest Management Science, 58, 859–867.
Grasso, V., Palermo, S., Sierotzki, H., Garibaldi, A., & Gisi, U. (2006). Cytochrome b gene structure and consequences for resistance to Qo inhibitor fungicides in plant pathogens. Pest Management Science, 62, 465–472.
Hibbett, D. S. (1992). Ribosomal RNA and fungal systematics. Transactions of the Mycological Society of Japan, 33, 533–556.
Hollomon, D. W., & Wheeler, I. E. (2002). Controlling powdery mildews with chemistry. In R. R. Bélanger, W. R. Bushnell, A. J. Dik, & T. L. W. Caver (Eds.), The powdery mildews. A comprehensive treatise (pp. 249–255). St. Paul: APS Press.
Holtschulte, B. (2000). Cercospora beticola – worldwide distribution and incidence. In: Asher, M.J.C., Holtschulte, B., Richard Molard, M., Rosso, F., Steinrucken, G., & Beckers, R. (Ed.), Cercospora beticola Sacc. Biology, Agronomic Influence and Control Measures in Sugar Beet, Advances in Sugar Beet Research, Vol. 2. (pp 5–16) Brussels: IIBR
Huang, X., & Madan, A. (1999). CAP3: a DNA sequence assembly program. Genome Research, 9, 868–877.
Hunte, C., Palsdottir, H., & Trumpower, B. L. (2003). Protonmotive pathways and mechanisms in the cytochrome bc 1 complex. FEBS Letters, 545, 39–46.
Jacobsen, B. J., & Franc, G. D. (2009). Cercospora leaf spot. In R. M. Harveson, L. E. Hanson, & G. L. Hein (Eds.), Compendium of beet diseases and pests (pp. 7–10). St. Paul, MN: APS Press.
Jiang, J., Ding, L., Michailides, T. J., Li, H., & Ma, Z. (2009). Molecular characterization of field azoxystrobin-resistant isolates of Botrytis cinerea. Pesticide Biochemistry and Physiology, 93, 72–76.
Joseph-Horne, T., & Hollomon, D. W. (1997). Molecular mechanisms of azole resistance in fungi. FEMS Microbiology Letters, 149, 141–149.
Karadimos, D. A., Karoglanidis, G. S., & Tzavella-Klonari, K. (2005). Biological activity and physical modes of action of the Q(o) inhibitor fungicides trifloxystrobin and pyraclostrobin against Cercospora beticola. Crop Protection, 24, 23–29.
Khan, M. F. R., & Smith, L. J. (2005). Evaluating fungicides for controlling Cercospora leaf spot on sugar beet. Crop Protection, 24, 79–86.
Khan, J., del Rio, L. E., Nelson, R., & Khan, M. F. R. (2007). Improving the Cercospora leaf spot management model for sugar beet in Minnesota and North Dakota. Plant Disease, 91, 1105–1108.
Kim, Y. S., Dixon, E. W., Vincelli, P., & Farman, M. L. (2003). Field resistance to strobilurin (QoI) fungicides in Pyricularia grisea caused by mutations in the mitochondrial cytochrome b gene. Phytopathology, 93, 891–900.
Kirk, W. W., Hanson, L. E., Franc, G. D., Stump, W. L., Gachango, E., Clark, G., et al. (2012). First report of strobilurin resistance in Cercospora beticola in sugar beet (Beta vulgaris) in Michigan and Nebraska, USA. New Disease Report, 26, 3.
Köller, W., & Wilcox, W. F. (1999). Evaluation of tactics for managing resistance of Venturia inaequalis to sterol demethylation inhibitors. Plant Disease, 83, 857–863.
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics, 23, 2947–2948.
Luo, C. X., Hu, M. J., Jin, X., Yin, L. F., Bryson, P. K., & Schnabel, G. (2010). An intron in the cytochrome b gene of Monilinia fructicola mitigates the risk of resistance development to QoI fungicides. Pest Management Science, 66, 1308–1315.
Ma, Z., & Michailides, T. J. (2005). Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Protection, 24, 853–863.
Ma, Z., Felts, D., & Michailides, T. J. (2003). Resistance to azoxystrobin in Alternaria isolates from pistachio in California. Pesticide Biochemistry and Physiology, 77(2), 66–74.
Malandrakis, A. A., Markoglou, A. N., Nikou, D. C., Vontas, J. G., & Ziogas, B. N. (2006). Biological and molecular characterization of laboratory mutants of Cercospora beticola resistant to Qo inhibitors. European Journal of Plant Pathology, 116, 155–166.
Meriggi, P., Rosso, F., Ioannidis, P.M., & Ayala Garcia, J. (2000) Fungicide treatments against Cercospora leaf spot in sugarbeet (Beta vulgaris L.). In: Asher, M.J.C., Holtschulte, B., Richard Molard, M., Rosso, F., Steinrücken, G., & Beckers, R. (Ed.) Cercospora beticola Sacc.– Biology, Agronomic Influence and Control Measures in Sugar Beet, Advances in Sugar Beet Research Vol. 2 (pp. 77–102) Brussels: IIBR
Miessner, S., & Stammler, G. (2010). Monilinia laxa, M. fructigena and M. fructicola: risk estimation of resistance to QoI fungicides and identification of species with cytochrome b gene sequences. Journal of Plant Diseases and Protection, 4, 162–167.
Miller, J., Rekoske, M., & Quinn, A. (1994). Genetic resistance, fungicide protection and variety approval policies for controlling yield losses from Cercospora leaf spot infection. J Sugar Beet Res, 31, 7–12.
Minagawa, N., & Yoshimoto, A. (1987). The induction of cyanide-resistant respiration in Hansenula anomala. Journal of Biochemistry, 101, 1141–1146.
Mizutani, A., Yukioka, H., Tamura, H., Miki, N., Masuko, M., & Takeda, R. (1995). Respiratory characteristics in Pyricularia oryzae exposed to a novel alkoxyiminoacetamide fungicide. Phytopathology, 85, 306–311.
Nei, M. (1973). Analysis of gene diversity in subdivided populations. Proceedings of the National Academy of Sciences of the United States of America, 70, 3321–3323.
Osyczka, A., Moser, C. C., & Dutton, P. L. (2005). Fixing the Q cycle. Trends in Biochemical Sciences, 30(4), 176–182.
Osyczka, A., Zhang, H., Mathé, C., Rich, P. R., Moser, C. C., & Dutton, P. L. (2006). Role of the PEWY glutamate in hydroquinone-quinone oxidation-reduction catalysis in the Qo site of cytochrome bc1. Biochemistry, 45, 10492–10503.
Ouchane, S., Agalidis, I., & Astier, C. (2002). Natural resistance to inhibitors of the ubiquinol cytochrome c oxidoreductase of Rubrivivax gelatinosus: sequence and functional analysis of the cytochrome bc1 complex. Journal of Bacteriology, 184, 3815–3822.
Rice, P., Longden, I., & Bleasby, A. (2000). EMBOSS: the European molecular biology open software suite. Trends in Genetics, 16, 276–277.
Rosenzweig, N., Hanson, L., Clark, G., Franc, G., Stump, W., Jiang, Q., et al. (2015). Use of PCR-RFLP analysis to monitor fungicide resistance in Cercospora beticola populations from sugarbeet (Beta vulgaris) in Michigan, United States. Plant Disease, 99, 355–362.
Sauter, H., Steglich, W., & Anke, T. (1999). Strobilurins: evolution of a new class of active substances. Angewandte Chemie (International Ed. in English), 38(10), 1328–1349.
Secor, G. A., Rivera, V. V., Khan, M. F. R., & Gudmestad, N. C. (2010). Monitoring fungicide sensitivity of Cercospora beticola of sugar beet for disease management decisions. Plant Disease, 94(11), 1272–1282.
Semar, M., Strobel, D., Koch, A., Klappach, K., & Stammler, G. (2007). Field efficacy of pyraclostrobin against populations of Pyrenophora teres containing the F129L mutation in the cytochrome b gene. Journal of Plant Diseases and Protection, 114(3), 117–119.
Shane, W. W., & Teng, P. S. (1992). Impact of Cercospora leaf spot on root weight, sugar yield, and purity of Beta vulgaris. Plant Disease, 76(8), 812–820.
Siah, A., Deweer, C., Morand, E., Reignault, P., & Halama, P. (2010). Azoxystrobin resistance of French Mycosphaerella graminicola strains assessed by four in vitro bioassays and by screening of G143A substitution. Crop Protection, 29(7), 737–743.
Siedow, J. N., & Moore, A. L. (1993). A kinetic model for the regulation of electron transfer through the cyanide-resistant pathway in plant mitochondria. BBA-Bioenergetics, 1142, 165–174.
Sierotzki, H., Parisi, S., Steinfeld, U., Tenzer, I., Poirey, S., & Gisi, U. (2000). Mode of resistance to respiration inhibitors at the cytochrome bc1 enzyme complex of Mycosphaerella fijiensis field isolates. Pest Management Science, 56(10), 833–841.
Sierotzki, H., Frey, R., Wullschleger, J., Palermo, S., Karlin, S., Godwin, J., et al. (2007). Cytochrome b gene sequence and structure of Pyrenophora teres and P. tritici-repentis and implications for QoI resistance. Pest Management Science, 63(3), 225–233.
Smith, G. A., & Martin, S. S. (1978). Differential response of sugarbeet cultivars to Cercospora leaf spot disease. Crop Science, 18(1), 39–42.
Smith, G. A., & Ruppel, E. G. (1971). Cercospora leaf spot as a predisposing factor in storage rot of sugar beet roots. Phytopathology, 61, 1485–1487.
Smith, G. A., & Ruppel, E. G. (1973). Association of Cercospora leaf spot, gross sucrose, percentage sucrose, and root weight in sugarbeet. Canadian Journal of Plant Science, 53(3), 695–696.
Stammler, G., Miessner, S., & Mann, W. (2011). Guignardia bidwellii, the causal agent of black rot on grapevine has a low risk for QoI resistance. Journal of Plant Diseases and Protection, 2(118), 51–53.
Torriani, S. F. F., Linde, C. C., & McDonald, B. A. (2009). Sequence conservation in the mitochondrial cytochrome b gene and lack of G143A QoI resistance allele in a global sample of Rhynchosporium secalis. Australasian Plant Pathology, 38(2), 202–207.
Vaghefi, N., Hay, F., Kikkert, J. R., & Pethybridge, S. J. (2016). Genotypic diversity and resistance to azoxystrobin of Cercospora beticola on processing table beet in New York. Plant Dis doi:10.1094/PDIS-09-15-1014-RE
Vallières, C., Trouillard, M., Dujardin, G., & Meunier, B. (2011). Deleterious effect of the Qo inhibitor compound resistance-conferring mutation G143A in the intron-containing cytochrome b gene and mechanisms for bypassing it. Applied and Environmental Microbiology, 77(6), 2088–2093.
Vanlerberghe, G. C., & McIntosh, L. (1997). Alternative oxidase: from gene to function. Annual Review of Plant Biology, 48(1), 703–734.
Veiga, A., Arrabaça, J. D., & Loureiro-Dias, M. C. (2003). Cyanide-resistant respiration, a very frequent metabolic pathway in yeasts. FEMS Yeast Research, 3(3), 239–245.
Weiland, J., & Koch, G. (2004). Sugarbeet leaf spot disease (Cercospora beticola Sacc.). Molecular Plant Pathology, 5(3), 157–166.
Widger, W. R., Cramer, W. A., Herrmann, R. G., & Trebst, A. (1984). Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b 6-f complex: position of the cytochrome b hemes in the membrane. Proceedings of the National Academy of Sciences of the United States of America, 81(3), 674–678.
Wood, P. M., & Hollomon, D. W. (2003). A critical evaluation of the role of alternative oxidase in the performance of strobilurin and related fungicides acting at the Qo site of complex III. Pest Management Science, 59(5), 499–511.
Zeng, F., Arnao, E., Zhang, G., Olaya, G., Wullschleger, J., Sierotzki, H., et al. (2015). Characterization of quinone outside inhibitor fungicide resistance in Cercospora sojina and development of diagnostic tools for its identification. Plant Disease, 99(4), 544–550.
Zhang, Z., Huang, L., Shulmeister, V. M., Chi, Y. I., Kim, K. K., Hung, L. W., et al. (1998). Electron transfer by domain movement in cytochrome bc 1. Nature, 392(6677), 677–684.
Zhang, Z., Schwartz, S., Wagner, L., & Miller, W. (2000). A greedy algorithm for aligning DNA sequences. Journal of Comparative Biology, 7(1–2), 203–214.
Ziogas, B. N., Baldwin, B. C., & Young, J. E. (1997). Alternative respiration: a biochemical mechanism of resistance to azoxystrobin (ICIA 5504) in Septoria tritici. Pesticide Science, 50(1), 28–34.
Zito, F., Finazzi, G., Joliot, P., & Wollman, F. A. (1998). Glu78, from the conserved PEWY sequence of subunit IV, has a key function in cytochrome b 6f turnover. Biochemistry, 37(29), 10395–10403.
Acknowledgments
This study was supported by the University of Wyoming Agricultural Experiment Station and Western Sugar Research Committee. Mention of trade names or commercial products in this article was solely for providing information and was neither a recommendation nor an endorsement of specific organizations mentioned.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
G. D. Franc, is deceased.
An erratum to this article is available at http://dx.doi.org/10.1007/s10658-016-1015-6.
Electronic supplementary material
Figure S1
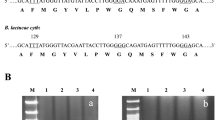
Alignment of the partial cytochrome b gene sequences from nine C. beticola isolates using ClustalW2 Seven QoI-sensitive isolates had identical cytochrome b gene sequences, whereas two QoI-resistant isolates (UW11–112 and UW11–117) had a single point mutation at codon 143 as shown in red (JPEG 374 kb)
Rights and permissions
About this article
Cite this article
Obuya, J.O., Franc, G.D. Molecular analysis of Cercospora beticola isolates for strobilurin resistance from the Central High Plains, USA. Eur J Plant Pathol 146, 817–827 (2016). https://doi.org/10.1007/s10658-016-0959-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-0959-x