Abstract
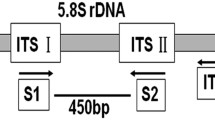
Ray blight, a destructive disease of Asteraceae worldwide, is caused by three morphologically similar but phylogenetically distinct species; Stagonosporopsis chrysanthemi, S. inoxydabilis and S. tanaceti. Stagonosporopsis chrysanthemi has been reported as a specific pathogen of chrysanthemum while S. inoxydabilis has been found associated with various Asteraceae. Stagonosporopsis tanaceti has only been reported in Australia, causing substantial crop loss on pyrethrum. All three species were shown to infect and cause disease on in vitro grown pyrethrum plants, hence, S. chrysanthemi and S. inoxydabilis may pose a significant biosecurity threat to the Australian pyrethrum industry. All these Stagonosporopsis species are also Level 2 quarantine pathogens in Europe. Rapid and accurate detection and differentiation of these species is a priority for ray blight management in Australia and in Europe. Accordingly, three species-specific PCR-based assays, targeted to the intergenic spacer of the nuclear ribosomal DNA, were developed. The specificity of each assay was confirmed against 21 Stagonosporopsis spp. as well as 14 pathogenic and saprophytic fungal species commonly found in association with pyrethrum in Australia. The primers were highly sensitive and specific to the target species, detecting down to 4 fg of genomic DNA. These primers were further used in a multiplex PCR to differentiate the presence of the three Stagonosporopsis spp. based on variable sized amplicons in a single reaction.






Similar content being viewed by others
References
Appel, D. J., & Gordon, T. R. (1996). Relationships among pathogenic and nonpathogenic isolates of Fusarium oxysporum based on the partial sequence of the intergenic spacer region of the ribosomal DNA. Molecular Plant-Microbe Interactions, 9(2), 125–138.
Baker, K. F., Dimock, A., & Davis, L. H. (1949). Life history and control of the Ascochyta ray blight of Chrysanthemum. Phytopathology, 39(10), 789–805.
Baker, K. F., Dimock, A., & Davis, L. H. (1961). Cause and prevention of rapid spread of Ascochyta disease of Chrysanthemum. Phytopathology, 51(2), 96.
BICON, 2016. Department of Agriculture and Water Resources Biosecurity Import Conditions Systems. Available online https://bicon.agriculture.gov.au/BiconWeb4.0.
Blakeman, J., & Hornby, D. (1966). The persistence of Colletotrichum coccodes and Mycosphaerella ligulicola in soil, with special reference to sclerotia and conidia. Transactions of the British Mycological Society, 49(2), 227–240.
Boerema, G. H., De Gruyter, J., Noordeloos, M., & Hamers, M. (2004). Phoma identification manual. Differentiation of specific and intra-specific taxa in culture. CABI, UK: Wallingford.
Brewer, M. T., Rath, M., & Li, H. (2015). Genetic diversity and population structure of the cucurbit gummy stem blight pathogen based on microsatellite markers. Phytopathology, 105(6), 815–824.
Chang, J. C., Hsu, M. M. L., Barton, R. C., & Jackson, C. J. (2008). High-frequency intragenomic heterogeneity of the ribosomal DNA intergenic spacer region in Trichophyton violaceum. Eukaryotic Cell, 7(4), 721–726.
Chesters, C., & Blakeman, J. (1966). The survival on chrysanthemum roots of epiphytic mycelium of Mycosphaerella ligulicola. Annals of Applied Biology, 58(2), 291–298.
Chesters, C., & Blakeman, J. (1967). Host range and variation in virulence of Mycosphaerella ligulicola. Annals of Applied Biology, 60(3), 385–390.
Chilvers, M. I., Du Toit, L. J., Akamatsu, H., & Peever, T. L. (2007). A real-time, quantitative PCR seed assay for Botrytis spp. that cause neck rot of onion. Plant Disease, 91(5), 599–608.
Chilvers, M. I., Jones, S., Meleca, J., Peever, T. L., Pethybridge, S. J., & Hay, F. S. (2014). Characterization of mating type genes supports the hypothesis that Stagonosporopsis chrysanthemi is homothallic and provides evidence that Stagonosporopsis tanaceti is heterothallic. Current Genetics, 60(4), 295–302.
De Gruyter, J., van Gent-Pelzer, M. P., Woudenberg, J. H., van Rijswick, P. C., Meekes, E. T., Crous, P. W., et al. (2012). The development of a validated real-time (TaqMan) PCR for detection of Stagonosporopsis andigena and S. crystalliniformis in infected leaves of potato and tomato. European Journal of Plant Pathology, 134(2), 301–313.
De Hoog, G. S., & Gerrits Van den Ende, A. H. G. (1998). Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses, 41(5–6), 183–189.
Dissanayake, M. L. M. C., Kashima, R., Tanaka, S., & Ito, S. I. (2009). Genetic diversity and pathogenicity of Fusarium oxysporum isolated from wilted welsh onion in Japan. Journal of General Plant Pathology, 75, 125–130.
EFSA PLH Panel (European Food Safety Authority Panel on Plant Health), 2013. Scientific opinion on the risks to plant health posed by Stagonosporopsis chrysanthemi (Stevens) Crous, Vaghefi and Taylor [Didymella ligulicola (Baker, Dimock and Davis) Arx var. ligulicola; syn. Didymella ligulicola (Baker, Dimock and Davis) Arx] in the EU territory, with identification and evaluation of risk reduction options. EFSA Journal, 11(10), 3376, 72 pp.
EPPO (2015). PQR-EPPO database on quarantine pests (available online). http://www.eppo.int
Ganley, A. R., & Scott, B. (1998). Extraordinary ribosomal spacer length heterogeneity in a Neotyphodium endophyte hybrid: implications for concerted evolution. Genetics, 150(4), 1625–1637.
Garibaldi, A., & Gullino, G. (1971). Brevi notizie sulla presenza in Italia dell’ascochitosi del crisantemo. L’Agricoltura Italiana, 71, 21–290.
Hay, F. S., Gent, D. H., Pilkington, S. J., Pearce, T. L., Scott, J. B., & Pethybridge, S. J. (2015). Changes in distribution and frequency of fungi associated with a foliar disease complex of pyrethrum in Australia. Plant Disease, 99(9), 1227–1235.
Hyde, K. D., Nilsson, R. H., Alias, S. A., Ariyawansa, H. A., Blair, J. E., Cai, L., et al. (2014). One stop shop: backbones trees for important phytopathogenic genera: I (2014). Fungal Diversity, 67(1), 21–125.
Jackson, C. J., Barton, R. C., Kelly, S. L., & Evans, E. G. V. (2000). Strain identification of Trichophyton rubrum by specific amplification of subrepeat elements in the ribosomal DNA nontranscribed spacer. Journal of Clinical Microbiology, 38(12), 4527–4534.
Jones, S. (2009). Characterisation of cultural, biological and molecular variability of Phoma ligulicola isolates associated with ray blight disease of pyrethrum and chrysanthemum, PhD thesis, University of Tasmania, Australia.
Katoh, K., & Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30(4), 772–780.
Kawabe, M., Kobayashi, Y., Okada, G., Yamaguchi, I., Teraoka, T., & Arie, T. (2005). Three evolutionary lineages of tomato wilt pathogen, Fusarium oxysporum f. Sp. lycopersici, based on sequences of IGS, MAT1, and pg1, are each composed of isolates of a single mating type and a single or closely related vegetative compatibility group. Journal of General Plant Pathology, 71(4), 263–272.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28(12), 1647–1649.
Latha, J., Chakrabarti, A., Mathur, K., Rao, V. P., Thakur, R. P., & Mukherjee, P. K. (2003). Genetic diversity of Colletotrichum graminicola isolates from India revealed by restriction analysis of PCR-amplified intergenic spacer region of nuclear rDNA. Current Science, 84(7), 881–883.
Liew, E., Maclean, D., & Irwin, J. (1998). Specific PCR based detection of Phytophthora medicaginis using the intergenic spacer region of the ribosomal DNA. Mycological Research, 102, 73–80.
Mirete, S., Patiño, B., Jurado, M., Vázquez, C., & González-Jaén, M. T. (2013). Structural variation and dynamics of the nuclear ribosomal intergenic spacer region in key members of the Gibberella fujikuroi species complex. Genome, 56(4), 205–213.
Muller, L. K., Lorch, J. M., Lindner, D. L., O’connor, M., Gargas, A., & Blehert, D. S. (2013). Bat white-nose syndrome: a real-time TaqMan polymerase chain reaction test targeting the intergenic spacer region of Geomyces destructans. Mycologia, 105(2), 253–259.
Nei, M., & Rooney, A. P. (2005). Concerted and birth-and-death evolution of multigene families. Annual Review of Genetics, 39, 121–152.
OEPP/EPPO (2010). PM 7/98 (1): specific requirements for laboratories preparing accreditation for a plant pest diagnostic activity. Bulletin OEPP/EPPO Bulletin, 40, 5–22.
Oxenham, B. L. (1963). Report of the plant pathology section. Australia: Report for the Department of Agriculture of Queensland.
Pantou, M. P., Mavridou, A., & Typas, M. A. (2003). IGS sequence variation, group-I introns and the complete nuclear ribosomal DNA of the entomopathogenic fungus Metarhizium: excellent tools for isolate detection and phylogenetic analysis. Fungal Genetics and Biology, 38(2), 159–174.
Peregrine, W., & Watson, D. (1964). Annual report of the plant pathology section. Tanganyika: Department of Agriculture.
Pethybridge, S. J., Hay, F., Clarkson, R., Groom, T., & Wilson, C. (2008a). Host range of Australian Phoma ligulicola var. inoxydablis isolates from pyrethrum. Journal of Phytopathology, 156(7–8), 506–508.
Pethybridge, S. J., Hay, F. S., Esker, P. D., Gent, D. H., Wilson, C. R., Groom, T., et al. (2008b). Diseases of pyrethrum in Tasmania: challenges and prospects for management. Plant Disease, 92(9), 1260–1272.
Pethybridge, S. J., Hay, F. S., & Groom, T. (2003). Seasonal fluctuations in fungi associated with pyrethrum foliage in Tasmania. Australasian Plant Pathology, 32(2), 223–230.
Pethybridge, S. J., Scott, J., & Hay, F. S. (2004). Genetic relationships among isolates of Phoma ligulicola from pyrethrum and chrysanthemum based on ITS sequences and its detection by PCR. Australasian Plant Pathology, 33(2), 173–181.
Pethybridge, S. J., & Wilson, C. (1998). Confirmation of ray blight disease of pyrethrum in Australia. Australasian Plant Pathology, 27(1), 45–48.
Pethybridge, S. J., Esker, P., Dixon, P., Hay, F., Groom, T., Wilson, C., et al. (2007). Quantifying loss caused by ray blight disease in Tasmanian pyrethrum fields. Plant Disease, 91(9), 1116–1121.
Pethybridge, S. J., Hay, F., Jones, S., Wilson, C., & Groom, T. (2006). Seedborne infection of pyrethrum by Phoma ligulicola. Plant Disease, 90(7), 891–897.
Rossi, V., Candresse, T., Jeger, M. J., Manceau, C., Urek, G., & Stancanelli, G. (2014). Diagnosis of plant pathogens and implications for plant quarantine: a risk assessment perspective. In M. L. Gullino & P. J. Bonants (Eds.), Detection and diagnostics of plant pathogens (pp. 167–193). Netherlands: Springer.
Rozen, S., & Skaletsky, H. (1999). Primer3 on the WWW for general users and for biologist programmers. In S. Misener & S. A. Krawetz (Eds.), Bioinformatics methods and protocols (pp. 365–386). New Jersey: Humana press.
Sampietro, D. A., Marín, P., Iglesias, J., Presello, D. A., Vattuone, M. A., Catalan, C. A. N., et al. (2010). A molecular based strategy for rapid diagnosis of toxigenic Fusarium species associated to cereal grains from Argentina. Fungal Biology, 114(1), 74–81.
Simmonds, J. H. (1996). Host index of plant disease in Queensland. Queensland Department of Primary Industries, Brisbane, 111.
Srinivasan, K., Gilardi, G., Spadaro, D., Garibaldi, A., & Gullino, M. L. (2011). Molecular characterization through IGS sequencing of formae speciales of Fusarium oxysporum pathogenic on lamb’s lettuce. Phytopathologia Mediterranea, 49(3), 309–320.
Stevens, F. L. (1907). The chrysanthemum ray blight. Botanical Gazette, 44(4), 241–258.
Stewart, J. E., Turner, A. N., & Brewer, M. T. (2015). Evolutionary history and variation in host range of three Stagonosporopsis species causing gummy stem blight of cucurbits. Fungal Biology, 119(5), 370–382.
Suarez, M. B., Walsh, K., Boonham, N., O’Neill, T., Pearson, S., & Barker, I. (2005). Development of real-time PCR (TaqMan®) assays for the detection and quantification of Botrytis cinerea in planta. Plant Physiology and Biochemistry, 43(9), 890–899.
Vaghefi, N., Ades, P. K., Hay, F. S., Pethybridge, S. J., Ford, R., & Taylor, P. W. J. (2015a). Identification of the MAT1 locus in Stagonosporopsis tanaceti, and exploring its potential for sexual reproduction in Australian pyrethrum fields. Fungal Biology, 119(5), 408–419.
Vaghefi, N., Hay, F. S., Ades, P. K., Pethybridge, S. J., Ford, R., & Taylor, P. W. J. (2015b). Rapid changes in the genetic composition of Stagonosporopsis tanaceti population in Australian pyrethrum fields. Phytopathology, 105(3), 358–369.
Vaghefi, N., Pethybridge, S. J., Ford, R., Nicolas, M. E., Crous, P. W., & Taylor, P. W. J. (2012). Stagonosporopsis spp. associated with ray blight disease of Asteraceae. Australasian Plant Pathology, 41(6), 675–686.
van der Aa, H., Noordeloos, M., & de Gruyter, J. (1990). Species concepts in some larger genera of the Coelomycetes. Studies in Mycology, 32, 3–19.
Walker, J., & Baker, K. F. (1983). The correct binomial for the chrysanthemum ray blight pathogen in relation to its geographical distribution. Transactions of the British Mycological Society, 80(1), 31–38.
Wang, Y., Hao, B., Zhang, Q., Tuo, E., Sun, G., Zhang, et al. (2012). Discovery of multiple IGS haplotypes within genotypes of Puccinia striiformis. Fungal Biology, 116(4), 522–528.
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungi ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR Protocols: a guide to methods and applications (pp. 315–322). San Diego: Academic.
Acknowledgments
We would like to thank Dr. Marin Talbot Brewer, University of Georgia, USA, for kindly providing DNA of Stagonosporopsis citrulli. We are also grateful to Ms. Azin Moslemi, University of Melbourne, Australia, for providing cultures of Fusarium spp. This project was supported by Botanical Resources Australia-Agricultural Services Pty. Ltd. The first author gratefully acknowledges the financial support from the Melbourne International Research Scholarship (MIRS) and Melbourne International Fee Remission Scholarship (MIFRS) awarded by the University of Melbourne.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(PDF 727 kb)
Rights and permissions
About this article
Cite this article
Vaghefi, N., Hay, F.S., Pethybridge, S.J. et al. Development of a multiplex PCR diagnostic assay for the detection of Stagonosporopsis species associated with ray blight of Asteraceae . Eur J Plant Pathol 146, 581–595 (2016). https://doi.org/10.1007/s10658-016-0944-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-0944-4