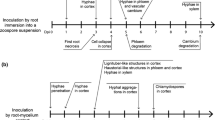
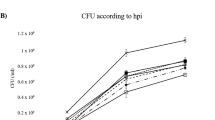
Abstract
The strong association between Phytophthora cinnamomi and the mortality and decline of Quercus suber and Q. ilex subsp. rotundifolia has been known for two decades. The ability of elicitins secreted by this pathogen to trigger defence responses in these Quercus against itself was evaluated in this work. Biomass quantification by quantitative real-time PCR revealed a significant decrease in pathogen colonization of Q. suber roots after 24 h pre-treatment with α- and β-cinnamomin. In Q. suber and Q. ilex roots pre-treated with α-cinnamomin, hyphae were unable to reach and colonize the vascular cylinder and showed cytoplasmic disorganization in all the roots observed as contrasted with non-pre-treated roots. The pathogen was restricted to the intercellular spaces of the cortical parenchyma and the concomitant accumulation of electron dense materials was observed in contact with the hyphae. Furthermore, ROS (reactive oxygen species) production and the enzymatic activities of superoxide dismutase, catalase and peroxidase were compared in infected and non-infected Quercus roots in time course trials. There was a significant increase in the production of hydrogen peroxide (H2O2) and superoxide anion (O2 •-) and an enhanced activity of the enzymes in infected roots was observed at each time point. When comparing with elicitin non-treated roots, the α-cinnamomin-treated roots in interaction with P. cinnamomi showed a decrease in ROS accumulation and an increase of the enzyme activities. The overall results were consistent with an induction by the cinnamomins which initiated defence responses against the pathogen invasion of roots. Finally, elicitins were immunolocalized in the contact zone of P. cinnamomi hyphae with epidermal host cells, plasmalemma outer cytoplasm and around the intracellular hyphae in the vacuoles of invaded epidermal cells.











Similar content being viewed by others
References
Able, A. J., Guest, D. I., & Sutherland, M. W. (1998). Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var nicotianae. Plant Physiology, 117, 491–499.
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Apel, K., & Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Reveiw Plant Biology, 55, 373–399.
Archer, M., Rodrigues, M. L., Aurelio, M., Biemans, R., Cravador, A., & Carrondo, M. A. (2000). Acta Crystallographica, D56, 363–365.
Benhamou, N., Bélanger, R. R., Rey, P., & Tirilly, Y. (2001). Oligandrin, the elicitin like protein produced by the mycoparasite Pythium oligandrum, induces systemic resistance to Fusarium crown and root rot in tomato plants. Plant Physiology and Biochemistry, 39, 681–698.
Beyer, W. F., & Fridovich, I. (1987). Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Analitical Biochemistry, 161, 559–566.
Blein, J. P., Coutos-Thévenot, P., Marion, D., & Ponchet, M. (2002). From elicitins to lipid-transfer proteins: a new insight in cell signalling involved in plant defense mechanisms. Trends Plant Science, 7, 293–296.
Bonnet, P., Bourdon, E., Ponchet, M., Blein, J.-P., & Ricci, P. (1996). Acquired resistance triggered by elicitins in tobacco and other plants. European Journal of Plant Pathology, 102, 181–192.
Brasier, C. M., Robredo, F., & Ferraz, J. F. P. (1993). Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathology, 42, 140–145.
Brummer, M., Arend, M., Fromm, J., Schlenzig, A., & Oβwald, W. F. (2002). Ultrastructural changes and immunocytochemical localization of the elicitin quercinin in Quercus robur L. roots infected with Phytophthora quercina. Physiological and Molecular Plant Pathology, 61, 109–120.
Caetano, P. (2007). Envolvimento de Phytophthora cinnamomi no declínio de Quercus suber e Q. rotundifolia: estudo da influência de factores bióticos e abióticos na progressão da doença. Possibilidades de controlo químico do declínio. PhD Dissertation, Universidade do Algarve, Portugal. http://sapientia.ualg.pt/handle/10400.1/400
Cakmak, I., & Marschner, H. (1992). Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiology, 98, 1222–1227.
Coelho, A. C., Horta, M., Neves, D., & Cravador, A. (2006). Involvement of a cinnamyl alcohol dehydrogenase of Quercus suber in the defence response to infection by Phytophthora cinnamomi. Physiological and Molecular Plant Pathology, 69, 62–72.
Crandall, B. S. (1950). The distribution and significance of the chestnut root rot Phytophthoras, P. cinnamomi and P. cambivora. Plant Disease Reporter, 34, 194–6.
Ebadzad, G., & Cravador, A. (2014). Quantitative RT-PCR analysis of differentially expressed genes in Quercus suber in response to Phytophthora cinnamomi infection. Springer Plus, 3, 613. doi:10.1186/2193-1801-3-613.
Eshraghi, L., Aryamanesh, N., Anderson, J. P., Shearer, B., McComb, J. A., Hardy, G. E. S. J., & O’Brien, P. A. (2011). A quantitative PCR assay for accurate in planta quantification of the necrotrophic pathogen Phytophthora cinnamomi. European Journal of Plant Pathology, 131, 419–430.
García-Pineda, E., Benezer-Benezer, M., Gutiérrez-Segundo, A., Rangel-Sánchez, G., Arreola-Cortés, A., & Castro-Mercado, E. (2010). Regulation of defence responses in avocado roots infected with Phytophthora cinnamomi. Plant and Soil, 331, 45–56.
Grant, R. B., Ebert, D., & Gayler, K. R. (1996). Elicitins: proteins in search of a role? Autralasian Plant Pathology, 25, 148–157.
Horta, M., Sousa, N., Coelho, A. C., Neves, D., & Cravador, A. (2008). In vitro and in vivo quantification of elicitin expression in Phytophthora cinnamomi. Physiological and Molecular Plant Pathology, 73, 48–57.
Horta, M., Caetano, P., Coelho, A. C., Medeira, C., Maia, I., Neves, D., & Cravador, A. (2010). Involvement of the β-cinnamomin elicitin in infection and colonisation of cork oak roots by Phytophthora cinnamomi. European Journal of Plant Pathology, 127, 427–436. doi:10.1007/s10658-010-9609-x.
Kamoun, S. (2006). A catalogue of the effector secretome of plant pathogenic oomycetes. Annual Review of Phytopathology, 44, 41–60.
Lamb, C., & Dixon, R. A. (1997). The oxidative burst in plant disease resistance. Annual Review of Plant Pathology and Plant Molecular Biology, 48, 251–275.
Lou, B., Wan, A., Lin, C. H., Xu, T., & Zhen, X. (2011). Enhancement of defence responses by oligandrin against Botrytis cinerea in tomatoes. African Journal of Biotechnology, 10(55), 11442–11449.
Manter, D. K., Kolodny, E. H., Hansen, E. M., & Parke, J. L. (2010). Virulence, sporulation and elicitin production in three clonal lineages of Phytophthora ramorum. Physiological and Molecular Plant Pathology, 74, 317–322.
Medeira, C., Quartin, V., Maia, I., Diniz, I., Matos, M. C., Semedo, J., Scotti-Campos, P., Ramalho, J., Pais, I., Ramos, P., Melo, E., Leitão, A., & Cravador, A. (2012a). Cryptogein and capsicein promote defence responses in Quercus suber against Phytophthora cinnamomi infection. European Journal of Plant Pathology, 134, 145–159. doi:10.1007/s10658-012-9972-x.
Medeira, C., Maia, I., Ribeiro, C., Candeias, I., Melo, E., Sousa, N., & Cravador, A. (2012b). Alpha cinnamomin elicit a defence response against Phytophthora cinnamomi in Castanea sativa. ISHS Acta Horticulturae, 940, 315–320.
Mikes, V., Milat, M.-L., Ponchet, M., Ricci, P., & Blein, J.-P. (1997). The fungal elicitor cryptogein is a sterol carrier protein. FEBS Letters, 416, 190–192.
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7, 405–410.
Mohamed, N., Lheirminier, J., Farmer, M.-J., Fromentin, J., Béno, N., Houot, V., Milat, M.-L., & Blein, J. P. (2007). Defense responses in grape vine leaves against Botrytris cinerea induced by application of a Pythium oligandrum strain or its elicitin, oligandrin, to roots. Phytopatology, 97, 611–620.
Oßwald, W., Fleischmann, F., Rigling, D., Coelho, A. C., Cravador, A., Diez, J., Dalio, R. J., Horta Jung, M., Pfanz, H., Robin, C., Sipos, G., Solla, A., Cech, T., Chambery, A., Diamandis, S., Hansen, E., Jung, T., Orlikowski, L. B., Parke, J., Prospero, S., & Werres, S. (2014). Strategies of attack and defence in woody plant-Phytophthora interactions. Forest Pathology, 44(3), 169–190. doi:10.1111/efp.12096.
Pires, N., Maia, I., Moreira, A., & Medeira, C. (2008). Early stages of infection of cork and holm oak trees by Phytophthora cinnamomi. In J. Vázquez & H. Pereira (Eds.), Suberwood: New challenges for the integration of cork oak forests and products (pp. 275–282). Spain: Universidad de Huelva.
Ponchet, M., Panabières, F., Milat, M. L., Mikes, V., Montillet, J. L., Suty, L., Triantaphylides, C., Tirilly, Y., & Blein, J. P. (1999). Are the elicitins cryptograms in plant-oomycete communications? Cellular and Molecular Life Sciences, 56, 1020–1047.
Pugin, A., & Guern, J. (1996). Mode of action of elicitors: involvement of plasma membrane functions. Comptes Rendus de l’Académie des Sciences, Série, 3(319), 1055–1061.
Ricci, P., Bonnet, P., Huet, J. C., Sallantin, M., Beauvais-Cante, F., Bruneteau, M., Billard, V., Michel, G., & Pernollet, J. C. (1989). Structure and activity of proteins from pathogenic fungi Phytophthora eliciting necrosis and acquired resistance in tobacco. European Journal of Biochemistry, 183, 555–563.
Robin, C., Desprez-Loustau, M.-L., Capron, G., & Delatour, C. (1998). First record of Phytophthora cinnamomi on cork and holm oaks in France and evidence of pathogenicity. Annual of Forest Science, 55, 869–883.
Rodrigues, M. L., Archer, M., Martel, P., Miranda, S., Thomaz, M., Enguita, F. J., Baptista, R. P., Melo, E. P., Sousa, N., Cravador, A., & Carrondo, M. A. (2006). Crystal structures of the free and sterol-bound forms of beta-cinnamomin. BBA-Proteins. Proteomics., 1764, 110–121.
Ruiz-Gómez, F. J., Navarro-Cerrillo, R. M., Sánchez-Cuesta, R., & Pérez-de-Luque, A. (2014). Histopathology of infection and colonization of Quercus ilex fine roots by Phytophthora cinnamomi. Plant Pathology. doi:10.1111/ppa.12310.
Sahoo, M. R., Das Gupta, M., Kole, P. C., Bhat, J. S., & Mukherjee, A. (2007). Antioxidative enzymes and isozymes analysis of taro genotypes and their implications in Phytophthora blight disease resistance. Mycopathologia, 163, 241–248.
Sánchez, M. E., Caetano, P., Ferraz, J., & Trapero, A. (2002). Phytophthora disease of Quercus ilex in south-western Spain. Forest Pathology, 32, 5–18.
Shearer, B. L., & Tippett, J. T. (1989). Jarrah Dieback: the dynamics and management of P. cinnamomi in the Jarrah (Eucalyptus marginata) Forest of South-western Australia. Department of conservation and land management. Western Australia: Research Bulletin, 3, 1–76.
van Kan, J. A. (2006). Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Science, 118(1), 247–253.
Van’t Slot, K. A. E., & Knogge, W. (2002). A dual role for microbial pathogen-derived effector proteins in plant disease and resistance. Critical Reviews in Plant Sciences, 21, 229–271.
Vance, C. P., Kirk, T. K., & Sherwood, R. T. (1980). Lignification as a mechanism of disease resistance. Annual Revivew Phytopathology, 18, 259–288.
Acknowledgments
This work was financed by the Portuguese Ministério da Ciência e do Ensino Superior (MCES) (PTCD/AGR-AAM/68628/2006). Ghazal Ebadzad thanks Fundação para a Ciência e a Tecnologia (FCT) for her grant (SFRH/BD/76979/2011) and Erasmus Mundus (EM8) program. The manuscript does not infringe any other person’s copyright or property rights.
Conflict of interest
The authors declare no conflicts of interest.
Compliance with ethical standards
The present research did not involve either animals or human participants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ebadzad, G., Medeira, C., Maia, I. et al. Induction of defence responses by cinnamomins against Phytophthora cinnamomi in Quercus suber and Quercus ilex subs. rotundifolia . Eur J Plant Pathol 143, 705–723 (2015). https://doi.org/10.1007/s10658-015-0721-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0721-9