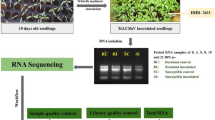
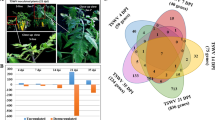
Abstract
Bacterial speck disease of tomato, caused by Pseudomonas syringae pv. tomato (Pst) is one of the most devastating diseases of tomato in the world. To investigate plant responses activated during plant-pathogen interaction, we studied the expression analysis of selected defense-response genes and the microbe or pathogen associated molecular patterns (MAMP or PAMP) triggered immunity (PTI) marker genes in heirloom tomatoes challenged with virulent Pst DC3000. Transcript levels of the defense response genes, including SlPR1a (pathogenesis related proteins), SlPR-Q′b (β-1,3-glucanase), SlGST (Glutathione S-transferase) and peroxidase were up-regulated in the resistant cultivars Orange Strawberry and Amishpaste. The PTI marker genes SlPTI5 (encode a pathogen-inducible ethylene response element-binding protein-like transcription factor) and SlFLS2 (a leucine rich-repeat receptor like kinase) were strongly up-regulated in the incompatible reaction to resistant cultivar Orange Strawberry. On the other hand, transcripts from all tested genes were down-regulated in the compatible interaction in susceptible cultivars Yellow Pear and Brandywine. The induction of defense response and PTI marker genes occurred in the early infection process at 3 days post-inoculation (dpi) and consistent with lower levels of disease severity in resistant cultivars was observed than in susceptible cultivars. Our results contribute to the body of information on bacterial speck disease resistance in an economically important crop tomato.



Similar content being viewed by others
References
Abel, S., & Theologis, A. (1996). Early genes and auxin action. Plant Physiology, 111, 9–17.
Alfano, J. R., & Collmer, A. (1996). Bacterial pathogens in plants: life up against the wall. The Plant Cell, 8, 1683–1698.
Apel, K., & Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology, 55, 373–399.
Asai, T., Tena, G., Plotnikova, J., Willmann, M. R., Chiu, W. L., Gomez-Gomez, L., Boller, T., Ausubel, F. M., & Sheen, J. (2002). MAP kinase signalling cascade in Arabidopsis innate immunity. Nature, 415, 977–983.
Ausubel, F. M. (2005). Are innate immune signaling pathways in plants and animals conserved? Nature Immunology, 6, 973–979.
Boch, J., Joardar, V., Gao, L., Robertson, T. L., Lim, M., & Kunkel, B. N. (2002). Identification of Pseudomonas syringae genes that are induced during infection of Arabidopsis thaliana. Molecular Microbiology, 44, 73–88.
Boller, T., & Felix, G. (2009). A renaissance of elicitors: perception of microbe associated molecular patterns and danger signals by pattern-recognition receptors. Annual Review of Plant Biology, 60, 379–406.
Boller, T., & He, S. Y. (2009). Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science, 324, 742–744.
Chambers, S. C., & Merriman, P. R. (1975). Perennation and control of Pseudomonas syringae pv. tomato. Australian Journal of Agricultural Research, 26, 657–663.
Dangl, J. L., Dietrich, R. A., & Richberg, M. H. (1996). Death don’t have no mercy: cell death programs in plant-microbe interactions. The Plant Cell, 8, 1793–1807.
Domingo, C., Conejero, V., & Vera, P. (1994). Genes encoding acidic and basic class III beta-1,3-glucanases are expressed in tomato plants upon viroid infection. Plant Molecular Biology, 24, 725–732.
Durrant, W. E., & Dong, X. (2004). Systemic acquired resistance. Annual Review of Phytopathology, 42, 185–209.
Ebel, J., & Cosio, E. G. (1994). Elicitors of plant defense responses. International Review of Cytology, 148, 1–36.
Foyer, C. H., & Noctor, G. (2005). Redox homeostis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell, 17, 1866–1875.
Gomez-Gomez, L., & Boller, T. (2000). FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Molecular Cell, 5, 1003–1011.
Gu, Y., & Martin, G. B. (1998). Molecular mechanisms involved in bacterial speck disease resistance of tomato. Philosophical Transactions e Royal Society London B Biological Science, 353, 1455–1461.
Hammond-Kosack, K. E., & Jones, J. D. G. (1996). Resistance gene-dependent plant defense responses. The Plant Cell, 8, 1773–1791.
Hann, D. R., & Rathjen, J. P. (2007). Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana. The Plant Journal, 49, 607–618.
Harvey, J. J., Lincoln, J. E., & Gilchrist, D. G. (2008). Programmed cell death suppression in transformed plant tissue by tomato cDNAs identified from an Agrobacterium rhizogenes-based functional screen. Molecular Genetics & Genomics, 279, 509–521.
He, P., Warren, R. F., Zhao, T., Shan, L., Zhu, L., Tang, X., & Zhou, J. M. (2001). Overexpression of Pti5 in tomato potentiates pathogen-induced defense gene expression and enhances disease resistance to Pseudomonas syringae pv. tomato. Molecular Plant-Microbe Interaction, 14, 1453–1457.
Hirano, S. S., & Upper, C. D. (2000). Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae: a pathogen, ice nucleus, and epiphyte. Microbiological & Molecular Biology Review, 64, 624–653.
Jones, J. D., & Dangl, J. L. (2006). The plant immune system. Nature, 444, 323.
Katagiri, F., Thilmony, R., & He, S. Y. (2002). The Arabidopsis thaliana-Pseudomonas syringae interaction. In C. R. Somerville & E. M. Meyerowitz (Eds.), The Arabidopsis book. American society of plant biologists (pp. 1–35). Rockville: Bio One Publishers.
Klement, Z., Rudolf, K., & Sands, D. (1990). Methods in Phytobacteriology. Budapest, Hungary Akademiai Kiado and Nyomda Vallalat.
Lamb, C., & Dixon, R. A. (1997). The oxidative burst in plant disease resistance. Annual Review Plant Physiology and Plant Molecular Biology, 48, 251–275.
Lu, D., Wu, S., Gao, X., Zhang, Y., Shan, L., & He, P. (2010). A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proceedings of National Academy of Science USA, 107, 496–501.
Martin, G. B., Brommonschenkel, S. H., Chunwongse, J., Frary, A., Ganal, M. W., Spivey, R., Wu, T., Earle, E. D., & Tanksley, S. D. (1993). Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science, 262, 1432–1436.
Monteiro, C. C., Carvalho, R. F., Gratao, P. L., Carvalho, G., Tezotto, T., Medici, L. O., Peres, L. E. P., & Azevedo, R. A. (2011). Biochemical responses of the ethylene insensitive Never ripe tomato mutant subjected to cadmium and sodium stresses. Environmental and Experimental Botany, 71, 306–320.
Nguyen, H. P., Chakravarthy, S., Velásquez, A. C., McLane, H. L., Zeng, L., Nakayashiki, H., Park, D. H., Collmer, A., & Martin, G. B. (2010). Methods to study PAMP-triggered immunity using tomato and Nicotiana benthamiana. Molecular Plant Microbe Interaction, 23, 991–999.
Passardi, C. F., Cosio, C., & Penel, C. (2005). Dunand Peroxidases have more functions than a Swiss army knife. Plant Cell Reports, 24, 255–265.
O’Brien, J. A., Daudi, A., Butt, V. S., & Bolwell, G. P. (2012). Reactive oxygen species and their role in plant defense and cell wall metabolism. Planta, 236, 765–779.
Pfaffl, M. W. (2001). A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Research, 29, e45.
Pitblado, R. E., & Kerr, E. A. (1980). Resistance to bacterial speck (Pseudomonastomato) in tomato. Acta Horticulturae, (Symposium on Production of Tomatoes for Processing)100, 379–82.
Pitblado, R. E., & MacNeill, B. H. (1983). Genetic basis of resistance to Pseudomonas syringae pv. tomato in the field tomatoes. Canadian Journal of Plant Pathology, 5, 251–255.
Sree Vidya, C. S., Manoharan, M., & Lakshmi Sita, G. (1999). Cloning and characterization of salicylic acid-induced, intracellular pathogenesis gene from tomato (Lycopersicon esculentum). Journal of Bioscience, 24, 287–293.
Tao, Y., Xie, Z., Chen, W., Glazebrook, J., Chang, H. S., Han, B., Zhu, T., Zou, G., & Katagiri, F. (2003). Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. The Plant Cell, 15, 317–330.
Thara, V. K., Gu, Y. Q., Tang, X., Martin, G. B., & Zhou, J. M. (1999). Pseudomonas syringae pv. tomato induces the expression of tomato EREBP-like genes Pti4 and Pti5 independent of ethylene, salicylate and jasmonate. The Plant Journal, 20, 475–483.
Tornero, P., Conejero, V., & Vera, P. (1994). A gene encoding a novel isoform of PR 1 protein family from tomato is induced upon viroid infection. Molecular General Genetics, 243, 47–53.
Uknes, S., Mauch-Mani, B., Moyer, M., Potter, S., Williams, S., Dincher, S., Chandler, D., Slusarenko, A., Ward, E., & Ryals, J. (1992). Acquired resistance in Arabidopsis. The Plant Cell, 4, 645–656.
Veluchamy, S., Hind, S. R., Dunham, D. M., Martin, G. B., & Panthee, D. R. (2014). Natural variation for responsiveness to flg22, flgII-28, and csp22 and Pseudomonas syringae pv. tomato in heirloom tomatoes. PLoS ONE, 9, e106119.
Vlot, A. C., Dempsey, D. A., & Klessig, D. F. (2009). Salicylic Acid, a multifaceted hormone to combat disease. Annual Review of Phytopathology, 47, 177–206.
Wen, N., Chu, Z., & Wang, S. (2003). Three types of defensive genes are involved in resistance to bacterial blight and fungal blast diseases in rice. Molecular Genetics & Genomics, 269, 331–339.
Yunis, H., Bashan, Y., Okon, H., & Henis, Y. (1980). Two sources of resistance to bacterial speck of tomato caused by Pseudomonas syringae pv. tomato. Plant Disease, 527, 851–852.
Zeng, W., & He, S. Y. (2010). A prominent role of the flagellin receptor FLAGELLIN SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiology, 153, 1188–1198.
Zhou, J., Tang, X., & Martin, G. B. (1997). The Pto kinase conferring resistance to tomato bacterial speck disease interacts with proteins that bind a cis-element of pathogenesis-related genes. The EMBO Journal, 16, 3207–3218.
Zipfel, C., Robatzek, S., Navarro, L., Oakeley, E., Jones, J. D. G., Felix, G., & Boller, T. (2004). Bacterial disease resistance in Arabidopsis through flagellin perception. Nature, 428, 764–767.
Acknowledgments
This study was supported by grants from National Science Foundation grant IOS-1025642 to Alan Collmer, PI, Gregory B. Martin and Dilip R. Panthee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veluchamy, S., Panthee, D.R. Differential expression analysis of a select list of genes in susceptible and resistant heirloom tomatoes with respect to Pseudomonas syringae pv. tomato . Eur J Plant Pathol 142, 653–663 (2015). https://doi.org/10.1007/s10658-015-0621-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0621-z