Abstract
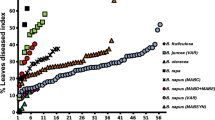
Field and controlled environment studies were undertaken to define the range and extent of available host resistances to Pseudocercosporella capsellae (white leaf spot) across diverse oilseed, forage and vegetable crucifers, including some wild and/or weedy species, and also within and/or derived from Brassica carinata. In each experiment, there was a wide range in host response from high resistance to high susceptibility as assessed by four disease parameters, viz. in the field for: (i) Area Under Disease Progress Curve (AUDPC) for percent leaves diseased with values ranging from 0 to 375.5; (ii) Percent Leaf Collapse Index (%LCI) for leaf collapse due to disease with values ranging from 0 to 23.0; and (iii), Percent Pod Area Disease Index (%PADI) for pod area affected with values ranging from 0 to 52.1; and (iv) under controlled environmental conditions for Percent Cotyledon Disease Index (%CDI) for cotyledon lesion size with values ranging from 0 to 27.5. At the Crawley field site, B. carinata ATC 94129 was the most resistant genotype with AUDPC = 1.2, followed by Crambe abyssinica (AUDPC 8.7), Eruca sativa Eruc-01 (AUDPC 19.3) and E. vesicaria Yellow rocket (AUDPC 19.4). B. carinata ATC 94129 and B. oleracea var. capitata had the least leaf collapse, with %LCI = 0.2. At the Shenton Park field site, 21 genotypes of B. carinata and B. oleracea var. acephala Tuscan kale showed total resistance, all with AUDPC values of 0. Of the B. napus genotypes carrying one or more B. carinata B genome introgressions, genotypes NC8 (AUDPC 23.0) and NC9-1 (AUDPC 26.2) were the most resistant. Genotypes as assessed on these disease criteria as having high level resistance generally showed no pod infection; in contrast to %PADI values up to 52 on the most susceptible genotypes. Under controlled environmental conditions, the most resistant genotype was B. carinata ATC 94129 with %CDI values of 0 and 0.2, respectively, across two experiments, along with B. napus genotypes Zhongyou 821 and Hyola 42, with a %CDI value of 0 in one of the two experiments. There was a high degree of correlation both within individual experiments across the different disease parameters and also between field and controlled environment experiments. Within both B. napus and B. juncea genotypes tested, the most resistant genotypes were from China, the most susceptible from India, with those from Australia intermediate.


Similar content being viewed by others
References
Amelung, D., & Daebeler, F. (1988). White spot (Pseudocercosporella capsellae Ell. et Ev. Deighton) - A new disease of winter rape in the German Democratic Republic. Nachrichtenblatt fuer den Pflanzenschutz in der DDR, 42, 73–74.
Barbetti, M. J., Banga, S. S., & Salisbury, P. A. (2012). Challenges for crop production and management from pathogen biodiversity and diseases under current and future climate scenarios – case study with oilseed Brassicas. Field Crops Research, 127, 225–240.
Barbetti, M. J., & Khangura, R. (2000). Fungal diseases of canola in Western Australia. Bulletin N, 4406, 15pp.
Barbetti, M. J., & Nichols, P. G. H. (2005). Field performance of subterranean clover germplasm in relation to severity of Cercospora disease. Australasian Plant Pathology, 34, 197–201.
Barbetti, M. J., & Sivasithamparam, K. (1981). Pseudocercosporella capsellae and Myrothecium verrucaria on rapeseed in Western Australia. Australasian Plant Pathology, 10, 43–44.
Boerema, G. H., & Verhoeven, A. A. (1980). Check-list for scientific names of common parasitic fungi. Series 2: Fungi on field crops: vegetables and cruciferous crops. European Journal of Plant Pathology, 86, 199–228.
Brun, H., & Tribodet, M. (1991). A method for estimate the level of resistance in oilseed rape to Pseudocercosporella capsellae. Bulletin OILB SROP, 14, 64–66.
Burdon, J., & Chilvers, G. (1982). Host density as a factor in plant disease ecology. Annual Review of Phytopathology, 20, 143–166.
Burton, W., Salisbury, P., & Potts, D. (2003). The potential of canola quality Brassica juncea as an oilseed crop for Australia. pp. 62–64, In: Proceedings of the 13th Australian Research Assembly on Brassicas Tamworth, NSW, Australia.
Campbell, C., & Madden, L. (1990). Temporal analysis of epidemics. I. Description and comparison of disease progress curves. pp. 161–202, In: Introduction to Plant Disease Epidemiology. New York: John Wiley & Sons.
Campbell, R., & Greathead, A. S. (1978). Pseudocercosporella white spot of crucifers in California. Plant Disease Reporter, 62, 1066–1068.
Cardone, M., Mazzoncini, M., Menini, S., Rocco, V., Senatore, A., Seggiani, M., et al. (2003). Brassica carinata as an alternative oil crop for the production of biodiesel in Italy: agronomic evaluation, fuel production by transesterification and characterization. Biomass and Bioenergy, 25, 623–636.
Cerkauskas, R. F., Stobbs, L. W., Lowery, D. T., Van Driel, L., Liu, W., & VanSchagen, J. (1998). Diseases, pests, and abiotic problems associated with oriental cruciferous vegetables in southern Ontario in 1993-1994. Canadian Journal of Plant Pathology, 20, 87–94.
Chen, S., Nelson, M. N., Ghamkhar, K., Fu, T., & Cowling, W. A. (2008). Divergent patterns of allelic diversity from similar origins: the case of oilseed rape (Brassica napus L.) in China and Australia. Genome, 51, 1–10.
Chen, S., Wan, Z., Nelson, M. N., Chuhan, J. S., Lin, P., Redden, R., et al. (2011). Two distinct genetic diversity groups of oilseed Brassica juncea in both China and India. pp. 20–23, In: Proceedings of the 17th Australian Research Assembly on Brassicas (ARAB), Wagga Wagga, NSW, Australia.
Crossan, D. F. (1954). Cercosporella leafspot of crucifers. North Carolina Agricultural Experiment Station Technical Bulletin, 109, 23.
Deighton, F. C. (1973). Studies on Cercospora and Allied Genera. Mycological Papers, 133, 42–46.
Dickson, M. H., & Petzoldt, R. (1993). Plant age and isolate source affect expression of downy mildew resistance in broccoli. HortScience, 28, 730–731.
Eshraghi, L., Barbetti, M. J., Li, H., Danehloueipour, N., & Sivasithamparam, K. (2007). Resistance in oilseed rape (Brassica napus) and Indian mustard (Brassica juncea) to a mixture of Pseudocercosporella capsellae isolates from Western Australia. Field Crops Reserch, 101, 37–43.
Eshraghi, L., You, M. P., & Barbetti, M. J. (2005). First report of white leaf spot caused by Pseudocercosporella capsellae on Brassica juncea in Australia. Plant Disease, 89, 1131–1131.
Garg, H., Sivasithamparam, K., Banga, S. S., & Barbetti, M. J. (2008). Cotyledon assay as a rapid and reliable method of screening for resistance against Sclerotinia sclerotiorum in Brassica napus genotypes. Australasian Plant Pathology, 37, 106–111.
Gudelj, I., Fitt, B. D. L., & Van den Bosch, F. (2004). Evolution of sibling fungal plant pathogens in relation to host specialization. Phytopathology, 94, 789–795.
Hind-Lanoiselet, T. L., Lewington, F., Wratten, K., Murray, G., & Edwards, J. (2003). Disease and drought-canola diseases in southern New South Wales and northern Victoria in 2002. pp. 111–115, In: Proceedings of the 13th Biennial Australian Reserch Assembly on Brassicas. Tamworth, Australia (NSW Department of Agriculture).
Inman, A. J. (1992). The biology and epidomiology of white leaf spot (Pseudocercosporella capsellae) on oilseed rape.,PhD thesis, The University of London,
Inman, A. J., Sivanesan, A., Fitt, B. D. L., & Evans, R. L. (1991). The biology of Mycosphaerella capsellae sp. nov., the teleomorph of Pseudocercosporella capsellae, cause of white leaf spot of oilseed rape. Mycological Reserch, 95, 1334–1342.
Lancaster, R. (2006). Diseases of Vegetable Brassicas. Farmnote 147, Department of Agriculture, Government of Western Australia.
Li, C., Li, H., Sivasithamparam, K., Fu, T., Li, Y., Liu, S., et al. (2006). Expression of field resistance under Western Australian conditions to Sclerotinia sclerotiorum in Chinese and Australian Brassica napus and Brassica juncea germplasm and its relation with stem diameter. Australian Journal of Agricultural Reserch, 57, 1131–1135.
Li, C. X., Li, H., Siddique, A. B., Sivasithamparam, K., Salisbury, P., Banga, S. S., et al. (2007). The importance of the type and time of inoculation and assessment in the determination of resistance in Brassia napus and B. juncea to Sclerotinia sclerotiorum. Australian Journal of Agricultural Research, 58, 1198–1203.
Li, C. X., Liu, S. Y., Sivasithamparam, K., & Barbetti, M. J. (2009). New sources of resistance to Sclerotinia stem rot caused by Sclerotinia sclerotiorum in Chinese and Australian Brassica napus and B. juncea germplasm screened under Western Australian conditions. Australasian Plant Patholology, 38, 149–152.
Malik, R. S. (1990). Prospects for Brassica carinata as an oilseed crop in India. Experimental Agriculture, 26, 125–129.
McKinney, H. H. (1923). Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. Journal of Agricultural Research, 26, 195–217.
Martinelli, J. A., Brown, J. K. M., & Wolfe, M. S. (1993). Effects of barley genotype on induced resistance to powdery mildew. Plant Pathology, 42, 195–202.
Miller, P. W., & McWhorter, F. P. (1948). A disease of cabbage and other crucifers due to Cercosporella brassicae. Phytopathology, 38, 893–898.
Monteiro, A. A., Coelho, P. S., Bahcevandziev, K., & Valério, L. (2005). Inheritance of downy mildew resistance at cotyledon and adult-plant stages in ‘Couve Algarvia’ (Brassica oleracea var. tronchuda). Euphytica, 141, 85–92.
Penaud, A. (1986). La maladie des taches blanches du colza causée par Pseudocercosporella capsellae. Information Techniques Cetiom, 95, 20.
Penaud, A. (1987). La maladie des taches blanches du colza. Phytoma, 95, 23–26.
Perron, G., & Nourani, D. (1991). Pseudocercosporella du colza: quantification du risque de contamination. pp. 639–645, In: Troisieme Conference Internationale sur les Malades Plantes 1991. Bordeaux, ANPP.
Perron, G., & Souliac, L. (1990). Pseudocercosporella capsellae (Et. et Ev) ou maladies des taches blanches du colza. La Defense des Vegetaux, 44, 22–27.
Petrie, G. A., Mortensen, K., & Dueck, J. (1985). Blackleg and other diseases of rapeseed in Saskatchewan, 1978 to 1981. Canadian Plant Disease Survey, 65, 35–41.
Petrie, G. A., & Vanterpool, T. C. (1978). Pseudocercosporella capsellae, the cause of white leaf spot and grey stem of cruciferae in Western Canada’. Canadian Plant Disease Survey, 58, 69–72.
Potts, D. A., Rakow, G. W., & Males, D. R. (1999). Canola-quality Brassica juncea, a new oilseed crop for the Canadian prairies. In: Proceedings of the 10 th International Rapeseed Congres Canberra, CD-ROM, Canberra, Australia
Rakow, G. (2004). Species origin and economic importance of Brassica. In E. C. Pua & C. Douglas (Eds.), Brassica (pp. 3–11). Verlag Berlin, Heidelberg, Germany: Springer.
Reyes, A. (1979). First occurrence of a severe white leafspot on Chinese mustard in Canada. Canadian Plant Disease Survey, 59, 1–2.
Rimmer, S. R., & Van den Berg, C. G. J. (1992). Resistance of oilseed Brassica spp. to blackleg caused by Leptosphaeria maculans. Canadian Journal of Plant Patholology, 14, 56–66.
Salisbury, P. A., & Barbetti, M. J. (2011). Breeding oilseed Brassica for climate change. In S. S. Yadav, R. T. Redden, J. S. Hatfield, H. Lotze-Campen, & A. Hall (Eds.), Crop adaptation to climate change (pp. 448–463). Chichester: Wiley.
Shivas, R. G. (1989). Fungal and bacterial diseases of plants in Western Australia. Journal of the Royal Society of Western Australia, 72, 1–62.
Snowdon, R., Lüchs, W., & Friedt, W. (2007). Brassica oilseeds. In R. J. Singh (Ed.), Genetic resources, chromosome engineering, and crop improvement (pp. 195–230). Boca Raton: CRC Press.
Sochting, H. P., & Verreet, J. A. (2004). Effects of different cultivation systems (soil management, nitrogen fertilization) on the epidemics of fungal diseases in oilseed rape (Brassica napus L. var. napus). Pflanzenkrankheiten und Pflanzenschutz, 111, 1–29.
Sumner, D. R., Glaze, N. C., Dowler, C. C., & Johnson, A. W. (1978). Foliar diseases of turnip grown for greens in intensive cropping systems. Plant Disease Reporter, 62, 51–55.
Uloth, M., You, M. P., Finnegan, P. M., Banga, S. S., Banga, S. K., Yi, H., et al. (2013). New sources of resistance to Sclerotinia sclerotiorum for crucifer crops. Field Crops Research, 154, 40–52.
Vale, F. X. R., Parlevliet, J. E., & Zambolim, L. (2001). Concepts in plant disease resistance. Fitopatologia Brasileira, 26, 577–589.
Acknowledgments
The first author gratefully acknowledges the financial assistance of an International SIRF Scholarship funded jointly by the Australian Government and The University of Western Australia. We appreciate the operational funding support for this research provided by the School of Plant Biology at The University of Western Australia. We thank Dr Huang Yi, Oil Crops Institute, China Academy of Agricultural Science, Wuhan, China for provision of seed lines with prefix YM from China; Dr Bob Redden, Curator, Australian Temperate Field Crops Collection, Horsham, Victoria for supplying seed lines with the ATC prefix; and Drs Phil Salisbury and Allison Gurung for also supplying some of the seed lines from the previous ACIAR program. We gratefully acknowledge the provision of half the salary of Martin Barbetti by the Department of Agriculture and Food Western Australia while these studies were undertaken.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunasinghe, N., You, M.P., Banga, S.S. et al. High level resistance to Pseudocercosporella capsellae offers new opportunities to deploy host resistance to effectively manage white leaf spot disease across major cruciferous crops. Eur J Plant Pathol 138, 873–890 (2014). https://doi.org/10.1007/s10658-013-0360-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-013-0360-y