Abstract
Diapause-specific peptide (DSP) is an insect antimicrobial peptide, composed of 41 amino acid residues including six cysteines and isolated from diapausing adults of the leaf beetle Gastrophysa atrocyaneais. Recent research results have been demonstrated that in vitro, DSP has selective antifungal activity against the higher animal pathogenic fungus Trichophyton rubrum and the plant phytopathogenic fungus Fusarium solani. To elucidate the effect of DSP on other phytopathogenic fungi, we respectively introduced a Cht1SP-DSP-FLAG fusion gene and Cht1SP-FLAG fusion gene into Arabidopsis ecotype col-0. Transgenic plants expressing Cht1SP-DSP-FLAG fusion gene showed significant resistance to Golovinomyces cichoracearum (biotrophic fungus), Botrytis cinerea (necrotrophic fungus) and Hyaloperonospora arabidopsidis (H. a.) Noco2 (oomycete), whilst transgenic plants expressing Cht1SP-FLAG fusion gene showed no resistance to these pathogens. These results indicated that for many plant pathogenic fungi, DSP has high antimicrobial activity, which suggests that DSP has a huge potential in protecting crops from damage from phytopathogenic fungi.





Similar content being viewed by others
References
Atiqur, R., Sharif, M. A., & Sun, C. K. (2011). Antifungal activity of essential oil and extracts of piper chaba hunter against phytopathogenic fungi. Journal of the American Oil Chemists’ Society, 88, 573–579. doi:10.1007/s11746-010-1698-3.
Clough, S. J., & Bent, A. F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. The Plant Journal, 16, 735–743. doi:10.1385/1-59745-130-4:87.
Coates, M. E., & Beynon, J. L. (2010). Hyaloperonospora arabidopsidis as a Pathogen Model. Annual Review of Phytopathology, 48, 329–345. doi:10.1146/annurev-phyto-080508-094422.
Consonni, C., Humphry, M. E., Hartmann, H. A., Livaja, M., Durner, J., Westphal, L., Vogel, J., Lipka, V., Kemmerling, B., Schulze-Lefert, P., Somerville, S. C., & Panstruga, R. (2006). Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nature Genetics, 38, 716–720. doi:10.1038/ng1806.
Daoubi, M., Hernandez-Galan, R., Benharref, A., & Collado, I. G. (2005). Screening study of lead compounds for natural product-based fungicides: antifungal activity and biotransformation of 6-alpha,7-alpha-dihydroxy betahimachalene by Botrytis cinerea. Journal of Agricultural and Food Chemistry, 53, 6673–6677. doi:10.1021/jf050697d.
Ferrari, S., Plotnikova, J. M., De Lorenzo, G., & Ausubel, F. M. (2003). Arabidopsis local resistance to Botrytis cinerea involves salicylic acid and camalexin and requires EDS4 and PAD2, but not SID2, EDS5 or PAD4. The Plant Journal, 35, 193–205. doi:10.1046/j.1365-313X.2003.01794.x.
Govrin, E. M., & Levine, A. (2000). The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Current Biology, 10, 751–757. doi:10.1016/S0960-9822(00)00560-1.
Hultmark, D., Steiner, H., Rasmuson, T., & Boman, H. G. (2008). Insect immunity.Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem, 106, 7–16.
Hwang, B., Hwang, J. S., Lee, J. Y., & Lee, D. G. (2010). Antifungal properties and mode of action of psacotheasin, a novel knottin-type peptide derived from Psacothea hilaris. Biochemical and Biophysical Research Communications, 3, 352–357. doi:10.1016/j.bbrc.2010.08.063.
Jansen, C., & Kogel, K. H. (2011). Insect antimicrobial peptides as new weapons against plant pathogens. Insect Biotechnology, Chapter 7, 123–144. doi:10.1007/978-90-481-9641-8_7.
Kouno, T., Mizuguchi, M., Tanaka, H., Yang, P., Mori, Y., Shinoda, H., Unoki, K., Aizawa, T., Demura, M., Suzuki, K., & Kawano, K. (2007). The structure of a novel insect peptide explains its Ca2+ channel blocking and antifungal activities. Biochemistry, 46, 13733–13741. doi:10.1021/bi701319t.
Li, X., Clarke, J. D., Zhang, Y., & Dong, X. (2001). Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Mol Plant Microbe In, 14, 1131–1139.
Makovitzki, A., Ada, V., Brotman, Y., Chet, I., & Shai, Y. (2007). Inhibition of fungal and bacterial plant pathogens in vitro and in planta with ultrashort cationic lipopeptides. Applied and Environmental Microbiology, 73, 6629–6636.
Montesinos, E. (2007). Antimicrobial peptides and plant disease control. FEMS Microbiology Letters, 270, 1–11. doi:10.1111/j.1574-6968.2007.00683.x.
Murray, M. G., & Thompson, W. F. (1980). Rapid isolation of high-molecular-weight plant DNA. Nucleic Acids Research, 8, 4321–4325. doi:10.1093/nar/8.19.4321.
Nie, H., Wu, Y., Yao, C., & Tang, D. (2011). Suppression of edr2-mediated powdery mildew resistance, cell death and ethylene-induced senescence by mutations in ALD1 in Arabidopsis. Journal of Genetics and Genomics, 38, 137–148. doi:10.1016/j.jgg.2011.03.001.
Otvos, L. (2000). Antibacterial peptides isolated from insects. Journal of Peptide Science, 6, 497–511. doi:10.1002/1099-1387(200010)6:10<497::AID-PSC277>3.0.CO;2-W.
Pan, H., Li, S., & Tang, D. (2011). HPR1, a component of the THO/TREX complex, plays an important role in disease resistance and senescence in Arabidopsis. The Plant Journal, 69, 831–843. doi:10.1111/j.1365-313X.2011.04835.x.
Russell, P. (1995). Fungicide resistance: occurrence and management. Journal of Agricultural Science, 124, 317–323. doi:10.1017/S0021859600073275.
Schägger, H. (2006). Tricine-SDS-PAGE. Nature Protocols, 1, 16–22. doi:10.1038/nprot.2006.4.
Schulze-Lefert, P., & Vogel, J. (2000). Closing the ranks to attack by powdery mildew. Trends in Plant Science, 5, 343–348. doi:10.1016/S1360-1385(00)01683-6.
Singh, A., & Banyal, D. K. (2011). Occurrence of different diseases of capsicum, tomato and cucumber under protected cultivation in Himachal Pradesh. Plant Disease Research, 26, 187–188.
Strange, R. N., & Scott, P. R. (2005). Plant disease: a threat to global food security. Annual Review of Phytopathology, 43, 83–116. doi:10.1146/annurev.phyto.43.113004.133839.
Tanaka, H., & Suzuki, K. (2005). Expression profiling of a diapause-specific peptide (DSP) of the leaf beetle Gastrophysa atrocyanea and silencing of DSP by double-strand RNA. Journal of Insect Physiology, 51, 701–707. doi:10.1016/j.jinsphys.2005.03.018.
Tanaka, H., Sato, K., Saito, Y., Yamashita, T., Agoh, M., Okunishi, J., Tachikawa, E., & Suzuki, K. (2003). Insect diapause-specific peptide from the leaf beetle has consensus with a putative iridovirus peptide. Peptides, 24, 1327–1333. doi:10.1016/j.peptides.2003.07.021.
Tang, D., Simonich, M., & Innes, R. W. (2007). Mutations in LACS2, a long-chain acyl-coenzymea synthetase, enhance susceptibility to avirulent pseudomonas syringae but confer resistance to Botrytis cinerea in Arabidopsis. Plant Physiology, 144, 1093–1103. doi:10.1104/pp.106.094318.
Thomma, B., Eggermont, K., Penninckx, I., Mauch-Mani, B., Vogelsang, R., Cammue, B. P. A., & Broekaert, W. F. (1998). Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proceedings of the National Academy of Sciences, 95, 15107–15111.
Wan, H., Yuan, M., You, H., Li, J. H., & Jin, B. R. (2012). Molecular cloning and characterization of a diapause-specific peptide in the beet armyworm, Spodoptera exigua. Journal of Asia-Pacific Entomology (online). doi:10.1016/j.aspen.2012.02.009.
Wang, Y., Nishimura, M. T., Zhao, T., & Tang, D. (2011). ATG2, an autophagy-related protein, negatively affects powdery mildew resistance and mildew-induced cell death in Arabidopsis. The Plant Journal, 68, 74–87. doi:10.1111/j.1365-313X.2011.04669.x.
Williamson, B., Tudzynski, B., Tudzynski, P., & Vankan, J. A. L. (2007). Botrytis cinerea: the cause of grey mould disease. Molecular Plant Pathology, 8, 561–580. doi:10.1111/J.1364-3703.2007.00417.x.
Zasloff, M. (2002). Antimicrobial peptides of multicellular organisms. Nature, 415, 389–395.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplemental Fig. 1
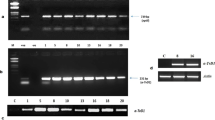
Cht1SP -FLAG fusion gene in Arabidosis transformants. A. The schematic representation of plant transformation construction of Cht1SP sequence. The synthetic Cht1SP was constructed into PGWB11 vector. RB, right border;LB,left border;HPT,hygromycin-resistance gene;35S,CaMV 35S promoter;Nos-T, terminator of the nopaline synthase gene;FLAG,FLAG tag. B. Detection of the introduced Cht1SP in transformants by genomic PCR. The size of Cht1SP sequence is 60 bp which a red arrow indicates. L plus numbers indicates the names of the transformant lines. M, DNA marker; Col-0, wild type control. C. Detection by real-time PCR of transcript of the introduced Cht1SP in transgenic plant lines (SL1, SL2, SL3, SL4, SL5, SL6, SL8). Relative gene expressions of Cht1SP in these transgenic lines and Col-0 wild type (as a control) were examined using real-time PCR with actin2 as an internal control. (JPEG 909 kb)

Supplemental Fig. 2
Transgenic Arabidopsis plants with Cht1SP-FLAG fusion gene showed no resistance to G.cichoracearum . Four-week-old plants were inoculated with G. cichoracearum. A. Leaves were stained with trypan blue at 7 dpi. Red arrow indicates an initiation colony. B. Quantification of fungal growth by calculating the number of conidiophores per colony at 7 dpi. Bars represent the mean (n > 30). Different letters indicate significant differences between transgenic lines and the wild type at P < 0.05. Experiments were repeated three times and the results are similar. (JPEG 1849 kb)

Supplemental Fig. 3
Transgenic Arabidopsis plants with Cht1SP-FLAG fusion gene exhibited no resistance to B. cinerea . A. Leaves from 4-week-old Col-0 and transgenic plants were detached and inoculated with B.cinerea. Leaves were photographed 3 dpi. B. Lesion size induced by B.cinerea. Lesion size was measured at 3 dpi. Bar represents the mean (n > 30) and SD from ten samples. Different letters indicate significant differences between transgenic lines and the wild type at (P < 0.05, t-test). Experiments were repeated two times and the results are similar. (JPEG 1561 kb)

Supplemental Fig. 4
Transgenic Arabidopsis plants with Cht1SP -FLAG fusion gene displayed no resistance to H. arabidopsidis. Two-week-old plants were infected with H. arabidopsidis. Noco2. The number of spores was monitored at 7 dpi. Bars represent the mean (n > 30) and standard deviation. Different letters indicates statistically significant differences between the samples (P < 0.05, t-test). Experiments were repeated three times and the results are similar. (JPEG 320 kb)
Rights and permissions
About this article
Cite this article
Wu, T., Chen, Y., Chen, W. et al. Transgenic expression of an insect diapause-specific peptide (DSP) in Arabidopsis resists phytopathogenic fungal attacks. Eur J Plant Pathol 137, 93–101 (2013). https://doi.org/10.1007/s10658-013-0219-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-013-0219-2