Abstract
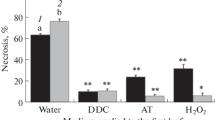
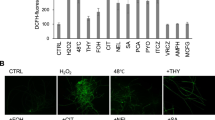
Cladosporium cucumerinum spore germination in vitro depended on spore suspension density. Different fungal isolates displayed germination maxima at different spore concentrations. For one isolate, the maximum was observed at the same spore density at both 18 and 25°C, although germination percentage increased slightly at the higher temperature. Diffusates originating in other spore suspensions of the same isolate reduced germination percentage of spores taken at optimal concentration. The least effect occurred in diffusate taken from spores kept at their optimal concentration. Self-suppression of spore germination at unfavourable concentrations was diminished more or less by antioxidants (superoxide dismutase, catalase, mannitol or formate). The same compounds, added to spore diffusates, reduced their fungitoxicities. All diffusates generated superoxide radical (assayed by adrenalin oxidation sensitive to superoxide dismutase). This activity correlated positively with diffusate toxicity. Leaf inoculation of the susceptible cucumber cultivar at 18°C with spore suspensions at extreme densities, at which they germinated poorly in vitro, led to less disease severity then that at optimal density. In contrast, no disease symptoms appeared at 25°C. It is suggested that spores germinating at their extreme concentrations produced reactive oxygen species, suppressing the pathogen; this effect could reduce disease development at low temperatures. At high temperatures, however, this mechanism seems not to work, suggesting that plant infection may be reduced by other disease inhibiting factors.






Similar content being viewed by others
Abbreviations
- CAT:
-
catalase
- CAT inactiv.:
-
catalase heat-inactivated
- O -2 :
-
superoxide anion radical
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
References
Aver’yanov, A. A., & Lapikova, V. P. (1990). Activated oxygen as a possible factor in the autoinhibition of spore germination of the fungus Pyricularia oryzae. Biokhimiya (Moscow), 55, 1397–1402.
Aver’yanov, A. A., Lapikova, V. P., & Dzhavakhiya, V. G. (1993). Active oxygen mediates heat-induced resistance of rice plant to blast disease. Plant Science, 92, 27–34.
Aver’yanov, A. A., Lapikova, V. P., Pasechnik, T. D., Kuznetsov, V. V., & Baker, C. J. (2007a). Suppression of early stages of fungus development by hydrogen peroxide at low concentrations. Plant Pathology Journal, 6, 242–247.
Aver’yanov, A. A., Pasechnik, T. D., Lapikova, V. P., Gaivoronskaya, L. M., Kuznetsov, V. V., & Baker, C. J. (2007b). Possible contribution of blast spores to the oxidative burst in the infection droplet on rice leaf. Acta Phytopathologica et Entomologica Hungarica, 42, 305–319.
Bors, W., Michel, C., Saran, M., & Lengfelder, E. (1978). The involvement of oxygen radicals during the autoxidation of adrenalin. Biochimica et Biophysica Acta, 540, 162–172.
Chitarra, G. S., Abee, T., Rombouts, F. M., Posthums, M. A., & Dijksterhuis, J. (2004). Germination of Penicillium paneum conidia is regulated by 1-octen-3-ol, a volatile self-inhibitor. Applied and Environmental Microbiology, 70, 2823–2829.
Czapski, G. (1984). On the use of ·OH scavengers in biological systems. Israel Journal of Chemistry, 24, 29–32.
Egan, M. J., Zheng-Yi, W., Jones, M. A., Smirnoff, N., & Talbot, N. J. (2007). Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proceedings of the National Academy of Sciences, USA, 104, 11772–11777.
Gäumann, E. (1951). Pflanzliche infectionslehre. Basel: Birkhaeuser.
Halliwell, B., & Gutteridge, J. M. C. (2007). Free Radicals in Biology and Medicine. Oxford: Oxford University Press.
Lapikova, V. P., Aver’yanov, A. A., & Pasechnik, T. D. (1995). Devices for the preparation of drop diffusates of cereal leaves. Mikologia i Fitopatologia (Mycology and Phytopathology, in Russian), 29, 44–47.
Lapikova, V. P., & Dzhavakhiya, V. G. (1987). Early development stages of Pyricularia oryzae Cav. on rice leaves. Mikologia i Fitopatologia (Mycology and Phytopathology, in Russian), 21, 358–365.
Leite, B., & Nicholson, R. L. (1992). Mycosporine-alanine: a self-inhibitor of germination from the conidial mucilage of Colletotrichum graminicola. Experimental Mycology, 16, 76–86.
Macko, V. (1981). Inhibitors and stimulators of spore germination and infection structure formation in fungi. In G. Turian & H. R. Hohl (Eds.), The fungal spore: Morphogenetic Controls (pp. 565–584). London-New York: Academic.
Peng, M., & Kuc, J. (1992). Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf discs. Phytopathology, 82, 696–699.
Ramakrishnan, L. (1967). Studies in the host-parasite relations of blast disease of rice. III. The effect of night temperature on the infection phase of blast disease. Phytopatologishe Zeitschrift, 57, 17–23.
Scott, B., & Eaton, C. J. (2008). Role of reactive oxygen species in fungal cellular differentiations. Current Opinion in Microbiology, 11, 488–493.
Tsurushima, T., Ueno, T., Fukami, H., Irie, H., & Inoue, M. (1995). Germination self-inhibitors from Colletotrichum gloesporioides f. sp. jussiaea. Molecular Plant-Microbe Interactions, 8, 652–657.
Acknowledgements
The work was partially supported by the grant 2682p of Agricultural Research Service USDA, mediated by International Science and Technology Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aver’yanov, A.A., Lapikova, V.P., Pasechnik, T.D. et al. Self-inhibition of spore germination via reactive oxygen in the fungus Cladosporium cucumerinum, causal agent of cucurbit scab. Eur J Plant Pathol 130, 541–550 (2011). https://doi.org/10.1007/s10658-011-9776-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-011-9776-4