Abstract
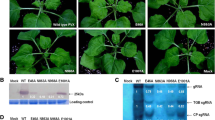
Papaya ringspot virus (PRSV) HA 5-1, a nitrous acid-induced mild mutant of severe strain HA, widely applied for control of PRSV by cross-protection, was used to study the genetic basis of attenuation. Using infectious clones, a series of recombinants was generated between HA 5-1 and HA and their infectivity was analyzed on the systemic host papaya and the local lesion host Chenopodium quinoa. The recombinants that contained mutations in P1 and HC-Pro genes caused attenuated infection on papaya without conspicuous symptoms, similar to HA 5-1. The recombination and sequence analyses strongly implicated two amino acid changes in the C-terminal region of P1 and two in HC-Pro of HA 5-1 involved in the attenuated infection on papaya. The recombinants that infected C. quinoa plants without local lesions contained the same mutations in the C-terminal region of HC-Pro for attenuated infection on papaya. We conclude that both P1 and HC-Pro bear important pathogenicity determinants for the infection on the systemic host papaya and that the mutations in HC-Pro affecting pathogenicity on papaya are also responsible for the inability to induce hypersensitive reaction on C. quinoa.






Similar content being viewed by others
References
Akira, K., Itaru, O., Kayoko, A., Yutaka, C., Shuu, H., Yoshiko, N. N., Akiko, I., & Yoshio, E. (1999). One amino acid change in cucumber mosaic virus RNA polymerase determines virulent/avirulent phenotypes on cowpea. Phytopathology, 89, 186–1192.
Arazi, T., Slutsky, S. G., Shiboleth, Y. M., Wang, Y., Rubinstein, M., Barak, S., Yang, J., & Gal-On, A. (2001). Engineering zucchini yellow mosaic potyvirus as a non-pathogenic vector for expression of heterologous proteins in cucurbits. Journal of Biotechnology, 87, 67–82.
Arbatova, J., Lehto, K., Pehu, E., & Pehu, T. (1998). Localization of the P1 protein of potato Y potyvirus in association with cytoplasmic inclusion bodies and in the cytoplasm of infected cells. Journal of General Virology, 79, 2319–2323.
Atreya, C. D., Atreya, P. L., Thornbury, D. W., & Pirone, T. P. (1992). Site-directed mutations in the potyvirus HC-Pro gene affect helper component activity, virus accumulation, and symptom expression in infected tobacco plants. Virology, 191, 106–111.
Atreya, C. D., & Pirone, T. P. (1993). Mutational analysis of the helper component-proteinase gene of a potyvirus: effects of amino acid substitutions, deletions, and gene replacement on virulence and aphid transmissibility. Proceedings of National Academy of Sciences USA, 90, 11919–11923.
Ballut, L., Drucker, M., Pugniere, M., Cambon, F., Blanc, S., Roquet, F., Candresse, T., Schmid, H. P., Nicolas, P., Gall, O. L., & Badaoui, S. (2005). HcPro, a multifunctional protein encoded by a plant RNA virus, targets the 20S proteasome and affects its enzymic activities. Journal of General Virology, 86, 2595–2603.
Brantley, J. D., & Hunt, A. G. (1993). The N-terminal protein of the polyprotein encoded by the potyvirus tobacco vein mottling virus is an RNA-binding protein. Journal of General Virology, 74, 1157–1162.
Brizard, J. P., Carapito, C., Delalande, F., Dorsselaer, A. V., & Brugidou, C. (2006). Proteome analysis of plant-virus interactome. Molecular and Cellular Proteomics, 5, 2279–2297.
Canto, T., Uhrig, J. F., Swanson, M., Wright, K. M., & MacFarlane, S. A. (2006). Translocaiton of Tomato bushy stunt virus P19 protein into the nucleus by ALY proteins compromises its silencing suppressor activity. Journal of Virology, 90, 9064–9072.
Chiang, C. H., & Yeh, S. D. (1997). Infectivity assays of in vitro and in vivo transcripts of papaya ringspot potyvirus. Botanical Bulletin of Academia Sinica, 38, 153–163.
Chu, M., Desvoyes, B., Turina, M., Noad, R., & Scholthof, H. B. (2000). Genetic dissection of tomato bushy stunt virus p19-protein-mediated host-dependent symptom induction and systemic invasion. Virology, 266, 79–87.
Chu, M., Lopez-Moya, J. J., Llave-Correas, C., & Pirone, T. P. (1997). Two separate regions in the genome of the tobacco etch virus contain determinants of the wilting response of Tabasco pepper. Molecular Plant-Microbe Interactions, 10, 472–480.
Fellers, J. P., Tremblay, D., Handest, M. F., & Lommel, S. A. (2002). The Potato virus Y MSNR NIb-replicase is the elicitor of a veinal necrosis-hypersensitive response in root knot nematode resistant tobacco. Molecular Plant Pathology, 3, 145–152.
Gal-On, A. (2000). A point mutation in the FRNK motif of the potyvirus helper component-protease gene alters symptom expression in cucurbits and elicits protection against the severe homologous virus. Phytopathology, 90, 467–473.
Gonsalves, D., & Ishii, M. (1980). Purification and serology of papaya ringspot virus. Phytopathology, 70, 1028–1032.
Jenner, C. E., Tomimura, K., Ohshima, K., Hughes, S. L., & Walsh, J. A. (2002). Mutations in Turnip mosaic virus P3 and cylindrical inclusion proteins are separately required to overcome two Brassica napus resistance genes. Virology, 300, 50–59.
Jenner, C. E., Wang, X., Tomimura, K., Ohshima, K., Ponz, F., & Walsh, J. A. (2003). The dual role of the potyvirus P3 protein of turnip mosaic virus as a symptom and avirulence determinant in brassicas. Molecular Plant-Microbe Interactions, 16, 777–784.
Kasschau, K. D., Cronin, S., & Carrington, J. C. (1997). Genome amplification and long-distance movement functions associated with the central domain of tobacco etch potyvirus helper component-proteinase. Virology, 228, 251–262.
Klein, P. G., Klein, R. R., Rodriguez-Cerezo, E., Hunt, A. G., & Shaw, J. G. (1994). Mutational analysis of the tobacco vein mottling virus genome. Virology, 204, 759–769.
Krause-Sakate, R., Redondo, E., Richard-Forget, F., Jadão, A. S., Houvenaghel, M. C., German-Retana, S., Pavan, M. A., Candresse, T., Zerbini, F. M., & Gall, O. L. (2005). Molecular mapping of the viral determinants of systemic wilting induced by a Lettuce mosaic virus (LMV) isolate in some lettuce cultivars. Virus Research, 109, 175–180.
Leonard, S., Plante, D., Wittmann, S., Daigneault, N., Fortin, M. G., & Laliberte, J. F. (2000). Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlated with virus infectivity. Journal of Virology, 74, 7730–7737.
Masuta, C., Nishimura, M., Morishita, H., & Hataya, T. (1999). A single amino acid change in viral genome-associated protein of potato virus Y correlates with resistance breaking in ‘Virgin A mutant’ tobacco. Phytopathology, 89, 118–123.
Merits, A., Guo, D., & Saarma, M. (1998). VPg, coat protein and five non-structural proteins of potato A potyvirus bind RNA in a sequence-unspecific manner. Journal of General Virology, 79, 3123–3127.
Omarov, R. T., Rezende, J. A., & Scholthof, H. B. (2004). Host-specific generation and maintenance of Tomato bushy stunt virus defective interfering RNAs. Molecular Plant-Microbe Interactions, 17, 195–201.
Paalme, V., Gammelgård, E., Järvekülg, L., & Valkonen, J. P. T. (2004). In vitro recombinants of two nearly identical potyviral isolates express novel virulence and symptom phenotypes in plants. Journal of General Virology, 85, 739–747.
Plisson, C., Drucker, M., Blanc, S., German-Retana, S., Le Gall, O., Thomas, D., & Bron, P. (2003). Structural characterization of HC-Pro, a plant virus multifunctional protein. Journal of Biological Chemistry, 278, 23753–23761.
Purcifull D. E., Edwardson J. R., Hiebert E., & Gonsalves, D. (1984). Papaya ringspot virus. Kew, UK: CMI/AAB Descriptions of Plant Viruses.
Redondo, E., Krause-Sakate, R., Yang, S. J., Lot, H., Le Gall, O., & Candresse, T. (2001). Lettuce mosaic virus pathogenicity determinants in susceptible and tolerant lettuce cultivars map to different regions of the viral genome. Molecular Plant-Microbe Interactions, 14, 804–810.
Rodriguez-Cerezo, E., Klein, P. G., & Shaw, J. G. (1991). A determinant of disease symptom severity is located in the 3′-terminal noncoding region of the RNA of a plant virus. Proceedings of National Academy of Sciences USA, 88, 9863–9867.
Ruffel, S., Dussault, M. H., Palloix, A., Moury, B., Bendahmane, A., Robaglia, C., & Caranta, C. (2002). A natural recessive resistance gene against potato virus Y in pepper corresponds to the eukaryotic initiation factor 4E (eIF4E). Plant Journal, 32, 1067–1075.
Saenz, P., Cervera, M. T., Dallot, S., Quiot, L., Quiot, J. B., Riechmann, J. L., & Garcia, J. A. (2000). Identification of a pathogenicity determinant of Plum pox virus in the sequence encoding the C-terminal region of protein P3 + 6K(1). Journal of General Virology, 81, 557–566.
Saenz, P., Quiot, L., Quiot, J. B., Candresse, T., & Garcia, J. A. (2001). Pathogenicity determinants in the complex virus population of a Plum pox virus isolate. Molecular Plant-Microbe Interactions, 14, 278–287.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual, 2nd ed. NY: Cold Spring Harbor Laboratory.
Scholthof, H. B., Scholthof, K., Kikkert, M., & Jackson, A. O. (1995). Tomato bushy stunt virus spread is regulated by two nested genes that function in cell-to-cell movement and host-dependent systemic invasion. Virology, 213, 425–438.
Simon-Buela, L., Guo, H. S., & Garcia, J. A. (1997). Long sequences in the 5′ noncoding region of plum pox virus are not necessary for viral infectivity but contribute to viral competitiveness and pathogenesis. Virology, 233, 157–162.
Suehiro, N., Natsuaki, T., Watanabe, T., & Okuda, S. (2004). An important determinant of the ability of Turnip mosaic virus to infect Brassica spp. and/or Raphanus sativus is in its P3 protein. Journal of General Virology, 85, 2087–2098.
Tribodet, M., Glais, L., Kerlan, C., & Jacquot, E. (2005). Characterization of Potato virus Y (PVY) molecular determinants involved in the vein necrosis symptom induced by PVYN isolates in infected Nicotiana tabacum cv. Xanthi. Journal of General Virology, 86, 2101–2105.
Ullah, Z., & Grumet, R. (2002). Localization of Zucchini yellow mosaic virus to the veinal regions and role of viral coat protein in veinal chlorosis conditioned by the zym potyvirus resistance locus in cucumber. Physiological and Molecular Plant Pathology, 60, 79–89.
Urcuqui-Inchima, S., Haenni, A. L., & Bernardi, F. (2001). Potyvirus proteins: a wealth of functions. Virus Research, 74, 157–175.
Verchot, J., & Carrington, J. C. (1995). Debilitation of plant potyvirus infectivity by P1 proteinase-inactivating mutations and restoration by second-site modifications. Journal of Virology, 69, 1582–1590.
Verchot, J., Herndon, K. L., & Carrington, J. C. (1992). Mutational analysis of the tobacco etch potyviral 35-kDa proteinase: identification of essential residues and requirements for autoproteolysis. Virology 190, 298–306.
Wang, C. H., & Yeh, S. D. (1992). Nucleotide sequence comparison of the 3′-terminal regions of severe, mild, and non-papaya infecting strains of papaya ringspot virus. Archives of Virology, 127, 345–354.
Wang, H. L., Wang, C. C., Chiu, R. J., & Sun, M. H. (1978). Preliminary study on papaya ringspot virus in Taiwan. Plant Protection Bulletin, 20, 133–140.
Yeh, S. D., & Gonsalves, D. (1984). Evaluation of induced mutants of papaya ringspot virus for control by cross protection. Phytopathology, 74, 1081–1085.
Yeh, S. D., Gonsalves, D., & Provvidenti, R. (1984). Comparative studies on host range and serology of papaya ringspot virus and watermelon mosaic virus 1. Phytopathology, 74, 1081–1085.
Yeh, S. D., Gonsalves, D., Wang, H. L., Nanba, R., & Chiu, R. J. (1988). Control of papaya ringspot virus by cross protection. Plant Disease, 72, 375–380.
Yeh, S. D., Jan, F. J., Chiang, C. H., Doong, T. J., Chen, M. C., Chung, P. H., & Bau, H. J. (1992). Complete nucleotide sequence and genetic organization of papaya ringspot virus RNA. Journal of General Virology, 73, 2531–2541.
Yoshii, M., Nishikiori, M., Tomita, K., Yoshioka, N., Kozuka, R., Naito, S., & Ishikawa, M. (2004). The Arabidopsis cucumovirus multiplication 1 and 2 loci encode translation initiation factors 4E and 4G. Journal of Virology, 78, 6102–6111.
Acknowledgements
The authors thank Drs. M. J. Chen, S. T. Hsu, and H. T. Hsu for their encouragement and advice. This research was partly supported by the grants NSC 87-2312-B-005-005 and NSC 89-2321-B-005-001 from the National Science Council of the Republic of China on Taiwan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiang, CH., Lee, CY., Wang, CH. et al. Genetic analysis of an attenuated Papaya ringspot virus strain applied for cross-protection. Eur J Plant Pathol 118, 333–348 (2007). https://doi.org/10.1007/s10658-007-9130-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-007-9130-z