Abstract
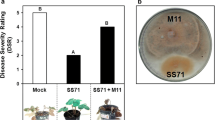
In the strawberry crop area of Tucumán (north-west Argentina) the three species of Colletotrichum causing anthracnose disease (C. acutatum, C. fragariae and C. gloeosporioides) were detected. Among all isolates characterized, one of them identified as C. acutatum (M11) and another as C. fragariae (F7) were selected due to their conspicuous interaction with the strawberry cultivar Pájaro. Whereas isolate M11 produced a strong compatible interaction in cv. Pájaro with clear disease symptoms (DSR = 5.0), the isolate F7 brought about a typical incompatible interaction (DSR = 1.0). When plants of cv. Pájaro were inoculated with F7 prior to the inoculation with M11, the former avirulent strain prevented the growth of the latter virulent pathogen. Experimental evidence indicated that the time elapsed between the first inoculation with the avirulent pathogen and the second inoculation with the virulent one was crucial to inhibit the growth of the latter. The growth of F7 on the plant without provoking damage and the fact that there was no in vitro antagonistic effect between the pathogens, suggests that the avirulent strain triggers a plant defensive response against M11. The defense response was further confirmed by the detection of an early oxidative burst occurring within 4 h after the first inoculation and by the observation of anatomical changes associated with defense mechanisms that lasted 50 days after the inoculation with F7. Results obtained support the hypothesis that the plant resistance against the virulent strain M11 is elicited by one or more diffusible(s) compound(s) produced by the avirulent strain F7.
Similar content being viewed by others
Abbreviations
- AOS:
-
Active Oxygen Species
- dai:
-
day after inoculation
- DSR:
-
Disease Severity Rating
- hai:
-
hour after inoculation
- RH:
-
Relative Humidity
- SEM:
-
Surface Electron Microscopy
References
Adaskaveg JE, Hartin RJ (1997) Characterization of Colletotrichum acutatum isolates causing anthracnose of almond and peach in California. Phytopathology 87: 979–987
Arias ME, Albornoz PL, Kirschbaum DS, Díaz Ricci JC, Castagnaro AP (2004) Foliar anatomy of species of the subtribe Potentillinae (Rosaceae) in Tucumán (Argentina). Boletín de la Sociedad Argentina de Botánica 38: 277–293
Brown GE (1978) Hypersensitive response of orange-colored Robinson tangerines to Colletotrichum gloeosporioides after ethylene treatment. Phytopathology 68: 700–706
Curry KJ, Abril M, Avant JB, Smith BJ (2002) Strawberry anthracnose: Histopathology of Colletotrichum acutatum and Colletotrichum fragariae. Phytopathology 92: 1055–1063
DeFosse L (1976) Recherches histochimiques sur la nature des reactions parietales formées en réponse á la penetration de Cercosporella herpotrichoides Fron dans le coleoptile du blé. Parasitica 32: 147–157
Delp BR, Milholland RD (1980) Evaluating strawberry plants for resistance to Colletotrichum fragariae. Plant Disease 64: 1071–1073
Denoyes B, Baudy A (1995) Species identification and pathogenicity study of french Colletotrichum strains isolated from strawberry using morphological and cultural characteristics. Phytopathology 85: 53–57
Denoyes-Rothan B, Lafargue M, Guérin G, Clerfeau M (1999) Fruit resistance to Colletotrichum acutatum in strawberries. Plant Disease 83: 549–553
Denoyes-Rothan B, Guérin G, Lerceteau-Köller E, Risser G (2005) Inheritance of resistance to Colletotrichum acutatum in Fragaria x ananassa. Phytopathology 95: 405–412
Dizeo de Strittmater C (1986) Manual de Técnicas en Histología Vegetal. In: Ana D’ Ambrogio de Argüeso (ed.) Chap. II. Hemisferio Sur, Buenos Aires Argentina
Doke N (1983) Generation of superoxide anion by potato tuber protoplasts during the hypersensitive response to hyphal wall components of Phytopthora infestans and specific inhibition of the reaction by suppressors of hypersensitivity. Physiological Plant Pathology 23: 359–367
Filippone MP, Díaz Ricci JC, Mamaní de Marchese A, Farías RN, Castagnaro AP (1999) Isolation and purification of a 316 Da preformed compound from strawberry (Fragaria ananassa) leaves active against plant pathogens. FEBS Letters 459: 115–118
Filippone MP, Díaz Ricci JC, Castagnaro AP, Farías RN (2001) Effect of fragarin on the cytoplasmic membrane of the phytopathogen Clavibacter michiganensis. Molecular Plant Microbe Interactions 14: 925–928
Freeman S, Katan T (1997) Identification of Colletotrichum species responsible for anthracnose and root necrosis of strawberry in Israel. Phytopathology 87: 516–521
Fulton RW (1986) Practices and precautions in the use of cross protection for plant virus disease control. Annual Review of Phytopathology 24: 67–81
Hammond KA, Jones JDG (1996) Resistance gene-dependent plant defense response. The Plant Cell 8: 1773–1791
Heath MC (1974) Chloroplast ultrastructure and ethylene production of senescing and rust-infected cowpea leaves. Canadian Journal of Botany 52: 2591–2597
Howard CM, Maas JL, Chandler CK, Albregts EE (1992) Anthracnose of strawberry caused by the Colletotrichum complex in Florida. Plant Disease 76: 976–981
Hudgins JW, Christiansen E, Franceschi VR (2004) Induction of anatomically based defense responses in stems of diverse conifers by methyl jasmonate: a phylogenetic perspective. Tree Physiology 24: 251–264
Kim KH, Yoon JB, Park HG, Park EW, Kim YH (2004) Structural modifications and programmed cell death of chili pepper fruit to resistance responses to Colletotrichuum gloeosporioides infection. Phytopathology 94: 1295–1304
Kuc J, Richmond S (1976) Aspect of the protection of cucumber against Colletotrichum lagenarium by Colletotrichum lagenarium. Phytopathology 67: 533–536
Kuc J (1982) Induced immunity to plant disease. Bio Science 32: 854–860
Kuc J (2001) Concepts and direction of induced systemic resistance in plants and its application. European Journal of Plant Pathology 107: 7–12
Kunoh H, Ishizaki H (1976) Accumulation of chemical elements around the penetration sites of Erysiphe graminis hordei on barley leaf epidermis. II. Level of silicon in papilla around the haustorial neck. Annals of the Phytopathological Society of Japan 42: 30–34
Lamb C and Dixon RA (1997) The oxidative burst in plant disease resistance. Annual Review of Plant Physiology and Plant Molecular Biology 48: 251–275
Lee YK, Hong JK, Hippe-Sanwald S, Hwang BK (2000) Histological and ultrastructural comparisons of compatible, incompatible and DL-β-amino-η-butyric acid-induced resistance responses of pepper stems to Phytophthora capsici Physiological and Molecular Plant Pathology 57: 269–280
Manandhar HK, Lyngs Jørgensen HJ, Mathur SB, Smedegaard-Petersen V (1998) Suppression of rice blast by preinoculation with avirulent Pyricularia oryzae and the nonrice pathogen Bipolaris sorokiniana Phytopathology 88: 735–739
Mayama S, Shishiyama J (1978) Localized accumulation of fluorescent and uv-absorbing compounds at penetration sites in barley leaves infected with Erysiphe graminis hordei. Physiological Plant Pathology 13: 347–354
Mena AJ, De Garcia EP, González MA (1974) Presencia de la antracnosis de la frutilla en la República Argentina. Revista Agronómica del Noroeste Argentino 11: 307–312
Métraux JP (2001) Systemic acquired resistance and salicylic acid: current state of knowledge. European Journal of Plant Pathology 107: 13–18
Mlodzianowski F, Siwecki R (1975) Ultrastructural changes in chloroplasts of Populus tremula L. leaves affected by the fungus Melampsora pinitorqua Braun. Physiological Plant Pathology 6: 1–3
Mónaco ME, Salazar SM, Aprea A, Díaz Ricci JC, Zembo JC, Castagnaro A (2000) First report of Colletotrichum gloeosporioides on strawberry in north-western Argentina. Plant Disease 84: 559
O’Connell RJ, Perfect S, Hughes B, Carzaniga R, Bailey JA, Gren J (2000) Dissecting the cell biology of Colletotrichum infection processes. In: Bailey JA, Jeger MJ (eds) Colletotrichum: Biology, Pathology, and Control. CAB International, Wallingford, UK, pp. 57–77
Ramallo CJ, Ploper LD, Ontivero M, Filippone MP, Castagnaro A, Díaz Ricci JC (2000) First report of Colletotrichum acutatum on strawberry in north-western Argentina. Plant Disease 84: 706
Robb JA, Harvey AE, Shaw M (1975) Ultrastructure of tissue cultures of Pinus monticola infected by Cronartium ribicola, II: Prepenetration host changes. Physiological Plant Pathology 5: 1–8
Ross AF (1961a) Systemic acquired resistance induced by localized virus infections in plants. Virology 14: 340–358
Ross AF (1961b) Localized acquired resistance to plant virus infection in hypersensitive hosts. Virology 14: 329–339
Ryals JA, Uknes S, Ward E (1994) Systemic acquired resistance. Plant Physiology 104: 1109–1112
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner H-Y, Hunt MD (1996) Systemic acquired resistance. The Plant Cell 8: 1809–1819
Sherwood RT, Vance CP (1976) Histochemistry of papillae formed in reed canarygrass leaves in response to noninfecting pathogenic fungi. Phytopathology 66: 503–510
Sherwood RT, Vance CP (1980) Resistance to fungal penetration in Gramineae. Phytopathology 70: 273–279
Shetty NP, Kristensen BK, Newman MA, Møller K, Gregersen PL, Jørgensen HJL (2003) Association of hydrogen peroxide with restriction of Septoria tritici in resistant wheat. Physiological and Molecular Plant Pathology 62: 333–346
Shishido M, Miwa C, Usami T, Amemiya Y, Johnson KB (2005) Biological control effficiency of Fusarium wilt of tomato by nonpathogenic Fusarium oxysporum Fo-B2 in different environments. Phytopathology 95: 1072–1080
Shoresh M, Yedidia I, Chet I (2005) Involvement of jasmonic acid/ethylene signaling pathway in the systemic resistance induced in cucumber by Trichoderma asperellum T203. Phytopathology 95: 76–84
Smith BJ, Black LL (1990) Morphological, cultural and pathogenic variation among Colletotrichum species isolated from strawberry. Plant Disease 74: 69–76
Staples RC, Hoch HC, Fevre P, Bourett T (1989) Heat shock induced development of infection structures by bean rust uredospore germlings. Experimental Mycology 13: 149–157
Sziráki I, Mustárdy L, Faludi-Dániel á, Király Z (1984) Alterations in chloroplast ultrastructure and chlorophyll content in rust-infected Pinto beans at different stages of disease development. Phytopathology 74: 77–84
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. The Plant Journal 11: 1187–1194
Wharton PS, Julian AM, O’Connell RJ (2001) Ultrastructure of the infection of Sorghum bicolor by Colletotrichum sublineolum. Phytopathology 91: 149–158
Xiao CL, Mackenzie SJ, Legard DE (2004) Genetic and pathogenic analyses of Colletotrichum gloeosporioides isolates fron strawberry and noncultivated hosts. Phytopathology 94: 446–453
Yun MH, Torres PS, Rigano LA, Oirdi ME, González-Lamothe R, Marano MR, Castagnaro AP, Dankert MA, Bouarab K, Vojnov AA (2006) Xanthan induces plant susceptibility by suppressing callose deposition. Plant Physiology 141: 178–187
Acknowledgements
This work was partially supported by the Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT, D346/1); Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET); Agencia Nacional de Promoción Científica y Tecnológica (UNT-PICTO 759). APC and JDR are members of CONICET. APC is also member of EEAOC.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Salazar, S.M., Castagnaro, A.P., Arias, M.E. et al. Induction of a defense response in strawberry mediated by an avirulent strain of Colletotrichum . Eur J Plant Pathol 117, 109–122 (2007). https://doi.org/10.1007/s10658-006-9075-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-006-9075-7