Abstract
This study successfully synthesized ZnO–CuO nanocomposite using the hydrothermal method with Carica papaya leaf extract. The incorporation of the leaf extract significantly enhanced the nanocomposite properties, a novel approach in scientific research. Characterization techniques, including X-ray diffraction, Fourier Transmission Infrared spectroscopy, and Scanning Electron Microscopy with Energy Dispersive X-Ray Analysis, confirmed a cubic crystal structure with an average size of 22.37 nm. The Fourier Transmission Infrared spectrum revealed distinctive vibrations at 627, 661, and 751 cm−1 corresponding to ZnO–CuO nanocomposite corresponding to stretching and vibration modes. SEM images confirmed a cubic-like and irregular structure. The nanocomposite exhibited outstanding photocatalytic activity, degrading methylene blue dye by 96.73% within 120 min under visible light. Additionally, they showed significant antimicrobial activity, inhibiting Staphylococcus aureus (20 mm) and Klebsiella pneumonia (17 mm). The results highlight the efficiency of Carica papaya leaf-derived ZnO–CuO nanocomposite for environmental and health challenges.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Research in the subject of nanotechnology is receiving significant attention globally in recent times. Many nanoparticles have potential uses in the culinary, pharmaceutical, medical, water treatment, and cosmetic industries due to their unique shape and crystallite size (Ghitman et al., 2020; Shanmugam et al., 2024; Usan et al., 2024). The metal nanoparticles offer a wide range of potential uses in industries such as photonics, electronics, catalysis, and the treatment of industrial and medical wastewater (TaghaviFardood et al., 2020). Several reports on the special qualities and practical uses of metal oxide nanocomposites made of gold, silver, palladium, iron, platinum, zinc, titanium, and other elements (Mahmoud et al., 2021). Metal oxide nanocomposite has shown incredible rest in various scientific disciplines. Among them, copper oxide (CuO) and zinc oxide (ZnO) nanocomposite garnered significant attention due to their unique properties (Akmaz et al., 2013; Ngullie et al., 2022). Zinc oxide (ZnO) nanoparticles are a type of nanoparticle that is biocompatible, multifunctional, and non-toxic. They are used in a wide range of industries, including sunscreens, antimicrobial agents used in food, coatings, paints, optics, and the removal of pollutants from aqueous media. Finally, they are used in medical fields, including drug delivery, gene delivery, biological activity, nanomedicine, and nanodiagnostics (Beura et al., 2021; Bocca et al., 2018; Kamburova et al., 2021; Rajivgandhi et al., 2021). ZnO nanostructures' high surface-to-volume ratio contributes to their appealing chemical and physical characteristics. Researchers have been paying close attention to ZnO because of its high catalytic activity, availability, stability, and biocompatibility. It is important to note that the increased surface area of ZnO nanostructure makes it more effective at photocatalysis and antibacterial activity than ZnOmicrosized particles (Beura et al., 2021; Kaviyarasu et al., 2017; Padmavathy & Vijayaraghavan, 2008). Since it is the foundation of various high temperature superconductors and giant magneto-resistance materials, as well as a p-type semiconductor with a tight band gap (1.2 eV in bulk), cupric oxide (CuO) has been the subject of intense study attention among transition metal oxides (MOs) (Anandan & Yang, 2007; Filipic & Cvelbar, 2012). Due to their potential for use in a wide range of applications, such as gas and biosensors, photodetectors and solar cells, nanofluids, supercapacitors, catalysis, near-infrared filters, magnetic storage media, and the preparation of different organic–inorganic nanocomposite, CuO nanostructures have been thoroughly studied (Jelani et al., 2018; Zhang et al., 2014). Since CuO nanoparticles exhibit antibacterial, antifungal, and antioxidant properties, they have found extensive usage in medicine. According to a recent paper, CuO nanoparticles target Methicillin resistant Staphylococcus aureus and E. Coli biofilms and stop the growth of infections in aquaculture (Chari et al., 2017). ZnO and CuO nanocomposite stood out for their distinct characteristics, including their applications in photoconductivity, photothermal processes, electronics, optics, nanofluid technology, and photonics. Copper and zinc oxide nanoparticles (CuO and ZnO NPs) have been of extensive interest because of the part of these nanoparticles in catalysis, biofuels, and wastewater treatment, environmental and biomedical applications (Bonthula et al., 2023; Farooq et al., 2019; Gayathri et al., 2021; Lu et al., 2017; Rajender et al., 2023; Ramyakrishna et al., 2022; Shanmugam et al., 2023). Researchers are increasingly turning to the green synthesis technique due to its use of less harmful chemicals, its eco-friendly nature, and its ability to facilitate one-stage synthesis of nanoparticles (Sundrarajan et al., 2015). Biosynthesis and environmental technologies are being employed to produceCuO and ZnONPs, which are considered to be non-toxic, environmentally safe, and biocompatible. A wide range of methods is available for the green synthesis of ZnO NPs (Lakshmeesha et al., 2014). In a study conducted by Jan et al. (2019) ZnO–CuO nanocomposite were synthesized using the hydrothermal method, achieving a remarkable 56% reduction in MB dye under UV–Vis illumination. Green synthesis is the process of creating nanoparticles by using natural substances as reducing or capping agents, such as plant extracts or biodegradable materials. This approach is centered on sustainability and mitigating the ecological consequences of nanoparticle manufacturing. In hydrothermal synthesis, these can include metal salts, organic compounds, or surfactants that break down or react under hydrothermal conditions to form nanoparticles. In green synthesis, reducing agents like polyphenols, flavonoids, terpenoids, and organic acids act as reducing agents to convert metal ions into nanoparticles. High pressure and temperature are used in an aqueous solution during hydrothermal synthesis to promote the development and production of nanoparticles. The synthesis takes place in a closed container known as a hydrothermal reactor or autoclave. The hydrothermal synthesis process may be made more environmentally friendly and sustainable by using green synthesis principles, such as the use of natural reducing agents or capping agents made from plant extracts or biodegradable materials. This technology decreases the environmental effect of classic hydrothermal synthesis methods and reduces dependency on chemical reducing agents. Through the integration of green synthesis principles with hydrothermal synthesis, scientists may generate functionalized nanoparticles with customized surface chemistries, rendering them appropriate for environmental remediation, sensing, and catalytic applications. Ardekani et al. (2018) reported that N-doped ZnO–CuO nanocomposite, prepared through ultrasonic spray pyrolysis, exhibited an impressive 80% degradation rate against MO dye. Rajith Kumar et al. (2020) described the combustion method to synthesize ZnO–CuO nanocomposite, effectively reducing MB dye. Govindasamy et al. (2021a) conducted a study on ZnO–CuO nanocomposite synthesized using a green approach, which demonstrated the inhibition of the growth of gram-positive and gram-negative bacteria, E. coli and S. aureus. Additionally, Taufiqueet et al. (2018) reported that chemically prepared ZnO–CuO nanocomposite exhibited strong antibacterial activity against S. aureus.The green synthesis of CuO, ZnO, and CuO/ZnO nanocomposite for use in biological applications using plant extracts is covered in a number of studies. ZnO NPs, CuO NPs, and CuO/ZnO NCs have been produced from a variety of plant extracts based on prior literature studies. It has been reported on the green production of CuO nanoparticles utilizing Syzygiumguineense (SyG) leaf extract on bacteria and the assessment of their electrochemical characteristics. The CuO/ZnO nanocomposite, which lowers the band gap energy and increases particle size, may be created by adding CuO to ZnO (Qamar et al., 2015). Penicilliumcorylophilum strain, a species of fungus, was combined with copper and zinc precursors to biosynthesize CuO/ZnO nanocomposite for photocatalytic activity. The ZnO and CuO nanoparticles are not as stable as the produced CuO/ZnO nanocomposite (Fouda et al., 2020a). The plant's biological uses have become excessive because of the existence of extremely powerful and therapeutic bioactive components. However, the present study to synthesis of novel nanocomposite in ZnO–CuO using an aqueous extract from Carica papaya leaf extracts and to reports its antibacterial activity against both human and food pathogens and photocatalytic activity against organic dye.
Materials and methods
Materials
The plant material of Carica papaya was composed from house garden, Junction, Salem, Tamil Nadu. The Sigma Aldrich chemicals (analytical grade) zinc acetate (Zn(CH3COO)2.2H2O; 99%) and copper acetate (Cu(CH3COO)2; 99%) were brought from Progen lab chemicals, Salem.
Synthesis of ZnO–CuO nanocomposite
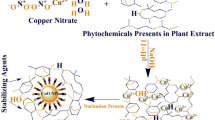
The stepwise procedure for the synthesis of biogenic ZnO and CuO nanocomposite is illustrated in Scheme 1. Synthesis of ZnO–CuO nanocomposite was followed slightly modified method by Bekru et al. (2022) was reported that, 5 g zinc acetate dissolved in 20 ml of double distilled water (DDW) and added with 20 ml of soxhlet extractor of Carica papaya leaf extract solution is a sample-A. In a 5 g copper acetate dissolved in 20 ml of DDW and added with 20 ml of leaf extract solution and it was marked as a sample-B. In a sample, A and B are mixed thoroughly and kept into magnetic stirring for 2 h at room temperature. After that sample was kept in hot air oven at 120 °C for 24 h. Then it was centrifuged at 5000 rpm for 15 min. In a supernatant and pellet are also separated in centrifuge tube after that supernatant is removed and are collected the pellet to pour into a petri dish. The poured petri dish was kept in a hot air oven at 24 h at 140 °C. Dried sample was collected, and it was transferred into cruciferous and using set a muffle furnace at 4 h at 350 °C. The phytochemicals found in the papaya leaf extract from Carica, including organic acids, polyphenols, flavonoids, and alkaloids, function as reducing agents to stabilize the CuO and ZnO nanoparticles during production. The mixture can be separated, cleaned, and dried for additional characterization after being heated to a certain temperature for a predetermined amount of time. This allows the reduction of Cu(II) ions to CuO nanoparticles and the same via the reduction of Zn(II) ions to ZnO nanoparticles.
Zn2+ + phytochemicals → ZnO + Reduced organic compounds.
Instrumentation studies
The diffraction pattern of the nanocomposite was recorded by the X-ray diffractometer (Rigaku Mini Flexll desktop) in 2θ range 10°–80° range. Morphological and elemental analysis was carried out by scanning electron microscope (SEM-Hitachi S3400n) with EDAX. Molecular structure and functional groups were studied by FTIR (Bruker alpha-p) with the spectrum range from 400–4000 cm−1. The optical properties were studied by Perkin Elmer Lambda 25 spectroscopy with absorption range from 200 – 800 nm.
Photocatalytic activity
The photocatalytic activity of ZnO–CuO nanocomposite was analyzed under solar light by evaluating the degradation of MB. About 20 mg of synthesized ZnO–CuO nanocomposite was mixed with 100 mL of MB aqueous solution. The mixture was stirred for 30 min. in dark conditions before irradiation. From that point forward, the suspension was presented to daylight illuminations. At various time stretches, barely any aliquots of color suspension were taken and centrifuged at 10,000 rpm for 10 min. and afterward absorbance range was estimated by UV-apparent spectroscopy (Liang et al., 2018). The level of photocatalytic debasement was determined from the following equation.
where C0—initial concentration, Ct—concentration at time (t).
Antibacterial activity assay
Synthesized ZnO–CuO nanocomposite was analysed against Gram + ve and Gram–vebacteria through agar disc diffusion method for antibacterial activity. Clinical isolated E. coli, S. aureus, K. pneumonia, and B. subtilis were sub-cultured used by nutrient broth at 24 h 37 °C. The different concentrations (25 µL, 35 µL, and 45 µL) of ZnO–CuO nanocompositeextract from the stock solution (mg/mL). The positive and negative controls were used in this study. Kanamycin used as a positive control. The plates were kept into hot air oven at 24 h for 37 °C. The zone of inhibition (ZOI) was measured after 24 h (Rajith Kumar et al., 2020).
Results and discussion
Synthesis of ZnO–CuO nanocomposite from Carica papaya
The present study, a biosynthesis of nanocomposite was prepared by using leaves of Carica papaya extract collected from home garden. The reduction of ZnO and CuO nanoparticles has been confirmed by visually manifested from the colour change after 24 h from green to dark brown (Fig. 1). The colour changes have been confirmed that the formation of ZnO–CuO nanocomposite. The synthesis of nanocomposite was confirmed by the observation of colour change. The CuO nanoparticles colour changed from yellow to dark green. And the synthesized ZnO nanoparticles colour changed from yellow to white. The synthesized ZnO–CuO nanocomposite colour changes to dark green (Jerry & Adeyemi. et al., 2022). The extract's bioactive components function as reducing agents, causing the Zn2+ and Cu2+ metal ions to be reduced to their corresponding metallic forms. The Carica papaya leaf extract contains phytochemicals that have two functions: they stabilize and reduce the freshly generated metal nanoparticles, so averting their uncontrollable agglomeration. In the extract's water, the reduced metal ions may first form metal hydroxides (Metal-OH). By stabilizing these metal hydroxides, the phytochemicals help to provide a regulated environment for further reactions. However, ZnO's absence of p-type electrical conductivity highlights how crucial it is to research hybrid heterojunctions. CuO is one of the better p-type materials to form p-n heterojunctions with ZnO, which has a wide range of applications in optoelectronic devices. CuO's nontoxicity, inexpensive market pricing, and high absorption coefficient are its primary benefits. In addition, there are numerous ways to prepare CuO, such as chemical bath deposition, thermal oxidation, magnetron sputtering, vacuum arc plasma evaporation, atomic layer deposition, electro deposition, ion sputtering, wet-chemical oxidation process, and spray pyrolysis. For use in photovoltaic systems, where CuO is used as an absorbing layer and wide band gap ZnO is used as a window layer, the combination of p-type CuO and n-type ZnO seems promising. Many kinds of gases, including CO, H2, volatile organic compounds, and H2S, have been effectively detected using CuO/ZnO junctions (Das & S. Vimal Chandra Srivastava. 2018; Yatskiv et al., 2019).
Structural studies
The crystallite size and structure of the as-prepared ZnO–CuO nanocomposite were characterized by XRD techniques. The XRD pattern of biosynthesized ZnO, CuO and ZnO–CuO nanocomposite from Carica papaya is show in Fig. 2a.The X-ray diffraction patterns of ZnO at 30.98°, 34.22°, 36.41°, 46.602°, 57.64°, 63.01°, 66.48°, and 67.97° corresponds to the (100), (002), (101), (102), (110), (103), (200), and (112) planes, respectively. These crystalline reflection data exhibit excellent agreement with the standard JCPDS 36-1451. Additionally, the observed diffraction peaks of CuO at 36.26, 37.94, 48.33, 54.13, and 59.01 values align well with the corresponding data values in the JCPDS 89-5895 dataset. Further, XRD pattern of ZnO/CuO composites materials containing all the diffraction peaks of ZnO and CuO. Thus, peak evidence confirmed the formation of ZnO/CuO nanocomposites (Klinbumrung et al., 2014). ZnO–CuO nanocomposite exhibited both phases of hexagonal and monoclinic structure of ZnO and CuO (Li & Wang, 2010; Yulizar et al., 2018). Moreover, the average crystalline size was calculated major intensity peaks using the Scherrer's equation. The 2θ, FWHM, and particle size values are given in the Table 1.
FTIR study
The surface functional groups, and formation of ZnO–CuO nanocomposite were confirmed by FTIR analysis and the corresponding spectra shown in Fig. 2b. The FTIR spectrum of Carica papaya extract exhibits peaks at 3185.25, 2879.35, 2834.67, 1590.10, 1412.13, 1112.17, 1016.58, and 951.13 cm−1. The robust absorption bands at 3185.25 and 2879.35 cm−1 are associated with the bending vibrations of C–H stretching groups in alkynes and H–C–H alkanes. Further, bands at 2834.67 and 1590 cm−1 are attributed to the H–C–H and N–H bending groups of amides, respectively. Moreover, the peaks at 1412.13 and 1112.17 cm−1 can be linked to the C–C=C asymmetric stretch group in aromatic rings and the aliphatic group in ethers. The band at 1016.58 cm−1 is assigned to the C=N stretching vibration of aliphatic amines (Bordbar et al., 2018; Preethi et al., 2020). The plant extract included polyphenolic chemicals, primarily phenolic acids, which functioned as both stabilizing and reducing agents to produce ZnO/Fe3O4 NCs, according to Fourier transform infrared (FTIR) spectroscopy data (Dwivedi et al., 2021; Govindasamy et al., 2021b). Furthermore, the peak observed at 627 cm−1 is indicative of Zn–O and Cu–O stretching vibrations. This peak evidence confirmed the formation of ZnO–CuO nanocomposites. Moreover, all the FTIR spectral evidence supports the plant extract was successfully coated onto the surface of ZnO–CuO nanocomposites.
Morphological studies
The surface structure of the nanocomposite was examined using SEM analysis (Fig. 2c, d).Its show the cubic and irregular shape of the nanocomposite. FEDAX analysis exhibits the presence of Cu, Zn, and O that’s proving the purity of the biosynthesized nanocomposite(Fig. 3). It was revealed of Zn (39.15%), Cu (19.82%), and O (32.99%). No impurities peaks were found, and it indicates the purity of biosynthesized nanocomposite. The present results were overlapped with previously reported work (Rajith Kumar et al., 2020).
Optical studies
The optical properties of the prepared ZnO–CuO nanocomposite were analyzed by DRS UV–Vis spectroscopy, and the corresponding images are shown in Fig. 4a. It can be observed that the prepared ZnO–CuO nanocomposites exhibit absorbance in the range from 300 to 450 nm. The ZnO–CuO nanocomposites show observed peaks that confirm the presence of ZnO and CuO (Koshimitsu et al., 2020). The observed peaks above 400 nm indicate that the prepared ZnO–CuO nanocomposites absorb visible light. The absorption of visible light favours the improvement of photocatalytic activity (Mohammadi-Aloucheh et al., 2018). Furthermore, based on the DRS UV–Visible spectrum, the energy band gap was calculated by Tauc plot (Fig. 4b). The calculated energy band gap values of the ZnO/CuO nanocomposite are 2.6 eV. Results from UV–Vis DRS verify the feasibility of the ZnO/CuO nanocomposite in the visible region.
Photocatalytic activity
The photocatalytic action of ZnO–CuO nanocomposite was inspected for MB colour under daylight light. The debasement absorbance of MB colour was estimated diverse time spans displayed in Fig. 5a. The maximum absorption nanocomposite peaks at near 670 nm for MB was noticed. The control experiment showed that MB does not degrade in the absence of photocatalysts, indicating that MB is more stable under these conditions. The degradation efficiency of the ZnO, CuO and ZnO/CuO nanocomposites for 56.21%, 42.32% and 96.73% of MB dye degradation activity (Tables 2 and 3). On comparing ZnO, CuO and ZnO/CuO nanocomposites, the ZnO/CuO composites shows better outstanding catalytic activity than ZnO and CuO nanoparticles. The composites materials prevent the electron hole pair recombination (Balaji et al., 2023; Thomas et al., 2022).The absorbance of dye decreases while increasing the time which indicate the very good dye degradation of ZnO–CuO nanocomposite. The photocatalytic activity of ZnO–CuO nanocomposite compared with other reported metal oxide nanoparticles was mentioned in Table 2. Sakib et al. have reported that, the ZnO–CuO nanocomposite was showed 91% of dye degradation against MB dye. Ullah et al. has studied that, the Cauliflower and potatoes peal wastes used to synthesizeZnO–CuO nanocomposite by facial chemical route for photocatalytic degradation applications. Based on these results, this prepared ZnO–CuO nanocomposite having higher MB dye degradation activity (Rajith Kumar et al., 2020; Sakib et al., 2019; Ullah et al., 2020).
The examination of the MB degradation kinetics for the prepared catalysts indicates the application of a first-order kinetic model to the experimental data. The equation − ln(C0/C) = kt was employed to determine the reaction rate of the sample, where C0 and C represents the initial and final concentration of MB, and k and t denote the rate constant and time, respectively. The linear correlation between ln(C0/C) and t illustrates the degradation of MB, conforming to pseudo-first-order kinetics for the prepared catalysts (Fig. 5c). The pseudo-first-order kinetic fit is evidently confirmed by the observed results. Based on the kinetic plot, rate constants (kobs) and correlation coefficients (R2) were calculated. The determined rate constants (kobs) and agreeing correlation coefficients (R2) indicate a substantial enhancement in dye degradation in the presence of ZnO–CuO nanocomposites. This suggests that the ZnO–CuO nanocomposites exhibit higher photocatalytic performance, revealing their effectiveness in preventing photocorrosion, suppress the recombination of electron hole pairs, and enhance active sites. Moreover, the recycling effectiveness of the prepared catalysts was assessed by subjecting them to the degradation of MB under consistent experimental conditions. Following each test, the catalysts were gathered using a centrifugation process, cleansed with deionized water, and subsequently dried in a hot air oven. The recycling assessment was performed over the course of five cycles under identical experimental conditions (Fig. 5d). Throughout these cycles, the degradation efficiency of each iteration remained consistent. This data indicates the commendable recycling properties of the ZnO–CuO nanocomposites.
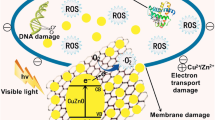
Potential mechanism for photocatalysis might be that ZnO and CuO nanoparticles absorb light, which sets off the degradation process. Because ZnO and CuO have similar bandgap energies, they can both absorb UV–visible photons and produce electron–hole pairs (e⁻-h⁺). Electrons (e⁻) in ZnO and CuO's valence band (VB) are stimulated to the conduction band (CB) upon light absorption, forming electron–hole pairs (e⁻-h⁺). These very reactive entities, known as electron–hole pairs, are essential to the degradation process. After reacting with molecular oxygen (O₂) or water (H₂O) adsorbed on the surface of the nanoparticles, the photogenerated electrons (e⁻) in the ZnO/CuO conduction band can produce superoxide radicals (·O₂⁻) or hydroxyl radicals (·OH), respectively. These radicals are potent oxidizers that may degrade organic compounds. The ZnO/CuO nanoparticles' surface is adsorbed with the dye molecules found in the solution. The adsorption stage is crucial because it brings the reactive species (·O₂⁻, ·OH) produced during the photocatalytic process near the dye molecules. The reactive species causes oxidation processes that break down the dye molecules once they have been adsorbed onto the surface of the nanoparticle.
Specifically, the ·OH radicals are very good at rupturing the chemical bonds that hold the dye molecules together, which causes the dye to degrade into less toxic, smaller compounds. Under perfect circumstances, the degradation process keeps going until all the dye molecules have calcified into inorganic compounds, such water (H2O), carbon dioxide (CO₂), and other simple molecules. The full eradication of dye contaminants from the environment depends on this mineralization process. In a nanocomposite, ZnO and CuO together can have synergistic effects that increase the photocatalytic activity. As an illustration, the heterojunction between ZnO and CuO nanoparticles can help with charge transfer and separation, increasing the dye degradation process's overall efficiency.
Antibacterial activity
Figure 6 shows the antibacterial activity of ZnO–CuO nanocomposite against G + ve and G-ve bacteria (E. coli, S. aureus, K. pneumonia, and B. subtilis) using disc diffusion method. The different concentrations were used from 25 to 45 µL. The nanocomposite displayed greater antibacterial activity against S. aureus (20 mm), K. pneumonia (17 mm) at a concentration 45 µL compared to positive control (Table 4). The evaluation of antibacterial movement used various metal oxides is shown in the Table 5. The antibacterial activity of ZnO–CuO nanocomposite wasshowing the high activity against S. aureus (Koshimitsu et al., 2020).Toenhances the meaningfulness of the antibacterial activity assessment, it is essential to conduct a dose–response analysis. This involves evaluating the antibacterial efficacy at various concentrations of the nanocomposite to identify an optimal concentration that exhibits effective antibacterial activity while minimizing toxicity. A microplate reader can be employed to assess bacterial growth inhibition or bacterial viability at different concentrations of ZnO–CuOnanocomposite. This approach allows for the generation of a dose–response curve, enabling the determination of the concentration at which antibacterial activity is maximized without causing undue harm.Moreover, defining a safe dose and understanding the potential cytotoxic effects on human cells are critical steps in assessing the applicability of these nanocomposite. It is advisable to explore the selective toxicity of the nanomaterial against bacterial cells while sparing mammalian cells. Incorporating these considerations and experimental approaches will enhance the relevance and safety assessment of the antibacterial activity of ZnO–CuO nanocomposite, providing valuable insights for their potential applications. The antibacterial activity of ZnO–CuO nanocomposite needs optimization and a dose–response analysis to determine a safe and effective concentration. Using a microplate reader, evaluating bacterial growth inhibition at various concentrations is crucial to identify the optimal antibacterial efficacy while minimizing toxicity. This approach ensures a more meaningful assessment and potential application of the nanocomposite.
Antibacterial and dye degradation possible mechanism
Antimicrobial and photocatalytic activities are inclined by limited basic factors like size, surface area, optical absorption, and morphology and separation performance of photo-generated charge carriers (Aizamddin et al., 2022; Murugan et al., 2016; Saravanan et al., 2013). The antibacterial activity is depending upon the reactive oxygen species (ROS), surface area, and size of the element. Figure 7 represents the antibacterial mechanism of synthesized ZnO–CuO nanocomposite. Nanoparticles and nanocomposite can act as photocatalysts and produce ROS such as •O2−,·OH, and H2O2 in the existence of dissolved O2 are important to extra free radical creation (Saleh & Gupta, 2011). The ZnO–CuO nanocomposite generate ROS thoroughly Fenton reaction leading to DNA damage, protein denaturation, finally it can reason for the death of the microorganism. This reaction is examined the.OH, and H2O2are dependable species for the photodegradation activity of MB dye molecule (Fig. 8; Eqs. (2)–(10)). Photocatalytic redox response by and large happens on the outside of impetuses and the upgraded surface properties significantly impact the proficiency of the photocatalyst (Arunadevi et al., 2018; Rangayasami et al., 2021).
The photocatalytic mechanism of MB over ZnO–CuO nanocomposites is illustrated in Fig. 8. It is essential to highlight that the photocatalytic reaction takes place on the surface of the photocatalyst. Heterogeneous photocatalysis by semiconductors requires fulfilment of two fundamental conditions: thermodynamic and kinetic. The formation of a heterojunction between CuO and ZnO facilitates the separation of photogenerated carriers (Klinbumrung et al., 2014). The energy band gap of the CuO and ZnO are 1.58 and 3.20 eV, respectively. In the presence of visible irradiation, CuO and ZnO are excited, resulting in the generation of electrons in the conduction band (CB) and holes in the valence band (VB), respectively, as depicted in Fig. 8. The band positions of the ZnO are below the CB and VB of CuO. The photoexcited e−1 transfer from CuO to ZnO, while the h+ migrate from ZnO to CuO. Subsequently, O2 molecules in the pollutant react with e−1 to produce superoxide radicals (·O2−), and the holes combine with H2O to generate hydroxyl radicals (·OH). Moreover, MB undergoes direct oxidation by the holes located at the valence band (VB) of CuO. The robust oxidant radicals of ·OH and ·O2− efficiently oxidize the MB molecule. We suggest the following plausible degradation mechanism for MB in the presence of sunlight using CuO/ZnO (Fouda et al., 2020b; Satdeve et al., 2019).
Conclusion
A novel ZnO–CuO nanocomposite in Carica papaya leaf extract was successfully synthesized through eco-friendlymethod. Leaf extract of Carica papaya extract have successfully mediated their composite due to stabilizing agent of phytochemical present in extract. The crystalline structure of nanocomposite was revealed using XRD analysis. The cubic like structure was determined by SEM analysis. Further, the ZnO–CuO photocatalyst shows outstanding photocatalytic efficiency of MB under visible light irradiation. Thee maximum degradation efficiency of 96.73% was achieved within 120 min. Moreover. Carica papaya plant extract stabilized ZnO–CuO nanocomposite shows better antibacterial agent against E. coli, S. aureus, K. pneumonia and B. subtilis. Furthermore, with the advantage of utilizing Carica papaya leaf extract to stabilize ZnO–CuO composites, the synthesis becomes environmentally friendly and cost-effective. The resulting flexible heterojunction ZnO–CuO composites offer promising prospects for applications such as adsorbents, electrochemical materials, and sensor support.
References
Aizamddin, M. F., Mahat, M., Zainal Ariffin, Z., Nawawi, M. A., Jani, N. A., Nor Amdan, N. A., & Sadasivuni, K. K. (2022). Antibacterial performance of protonated polyaniline- integrated polyester fabrics. Polymers, 14, 2617. https://doi.org/10.3390/polym14132617
Akmaz, S., DilaverAdiguzel, E., Yasar, M., & Erguven, O. (2013). The effect of Ag content of the chitosan silver nanoparticle composite material on the structure and antibacterialactivity. Advances in Materials Science and Engineering, 6, 690918.
Anandan, S., & Yang, S. (2007). Emergent methods to synthesize and characterize semiconductor CuO nanoparticles with various morphologies–an overview. Journal of Experimental Nanoscience, 2, 23–56.
Ardekani, S. R., Roughaghdam, A. S., & Nazari, M. (2018). N-doped ZnO–CuO nanocomposite prepared by one-step ultrasonic spray pyrolysis and its photocatalytic activity. Chemical Physics Letters, 705, 19–22.
Arunadevi, R., Kavitha, B., Rajarajan, M., Suganthi, A., & Jeyamurugan, A. (2018). Investigation of the drastic improvement of photocatalytic degradation of Congo red by monoclinic Cd, Ba-CuO nanoparticles and its antimicrobial activities. Surface Interfaces, 10, 32–44.
Bekru, A. G., Tufa, L. T., Zelekew, O. A., Goddati, M., Lee, J., & Sab, F. K. (2022). Green Synthesis of a CuO–ZnO nanocomposite for efficient photodegradation of methylene blue and reduction of 4-nitrophenol. ACS Omega, 7(35), 30908–30919.
Beura, R., Pachaiappan, R., & Paramasivam, T. (2021). Photocatalytic degradation studies of organic dyes over novel Ag-loaded ZnO graphene hybrid nanocomposite. Journal of Physics and Chemistry of Solids, 148, 109689.
Bharathi, D., Ranjithkumar, R., Chandrashekar, B., & Bhuvaneswari, V. (2019). Preparation of chitosan zinc oxide nanocomposite for enhanced antibacterial and photocatalytic activity: As a bionanocomposite. International Journals of Biological Macromolecules, 15(129), 989–996.
Bocca, B., Caimi, S., Senofonte, O., Alimonti, A., & Petrucci, F. (2018). ICPMS based methods to characterize nanoparticles of TiO2 and ZnO in sunscreens with focus on regulatory and safety issues. Science of the Total Environment, 630, 922–930.
Bonthula, S., Bonthula, S., Pothu, R., Srivastava, R. K., Boddula, R., Radwan, A. B., & Qahtani, N. A. (2023). Recent advances in copper-based materials for sustainable environmental applications. Sustainable Chemistry, 4, 246–271.
Bordbar, M., Negahdar, N., & Nasrollahzadeh, M. (2018). Melissa Officinalis L. leaf extract assisted green synthesis of CuO/ZnO nanocomposite for the reduction of 4- nitrophenol and Rhodamine B. Separation and Purification Technology, 191, 295–300.
Chari, N., Felix, L. O., Davoodbash, M. A., Ali, A., & A., Nooruddin, T. (2017). In vitro and in vivo antibiofilm effect of copper nanoparticle against aqua culture pahthogens. Biocatalysis and Agricutural Biotechnology, 10, 336–341.
Das, S., & Srivastava, V. C. (2018). An overview of the synthesis of CuO- ZnO nanocomposite for environmental and other applications. Nanotechnology Reviews, 7(3), 267–282.
Dwivedi, P., Jatrana, I., Khan, A. U., Khan, A. A., Satiya, H., Khan, M., Moon, S., & Alam, M. (2021). Photoremediation of methylene blue by biosynthesized ZnO/Fe3O4nanocomposites using Callistemon viminalis leaves aqueous extract: A comparative study. Nanotechnology Reviews, 10, 1912–1925.
Farooq, M. H., Aslam, I., Sadia Anam, H., Tanveer, M., Ali, Z., Ghani, U., & Rajender, B. (2019). Improved photocatalytic performance of reduced zinc oxide (ZnO) novel morphology of Astray like Microstructure under solar light irradiation. Materials Science for Energy Technologies, 2, 181–186.
Filipic, G., & Cvelbar, U. (2012). Copper oxide nanowires: A review of growth. Nanotechnology, 23(19), 194001.
Fouda, A., Salem, S. S., Wassel, A. R., Hamza, M. F., & Shaheen, T. I. (2020a). Optimization of green biosynthesized visible light active CuO/ZnOnano-photocatalysts for the degradation of organic methylene blue dye. Heliyon, 6, 9.
Fouda, A., Salem, S. S., Wassel, A. R., Hamza, M. F., & Shaheen, T. (2020b). Optimization of green biosynthesized visible light active CuO/ZnOnano-photocatalysts for the degradation of organic methylene blue dye. Heliyon, 6, e04896.
Ganesan, K., Jothi, V. K., Natarajan, A., Rajaram, A., Ravichandran, S., & Ramalingam, S. (2020). Green synthesis of copper oxide nanoparticles decorated with graphene oxide for anticancer activity and catalytic applications. Arabian Journal of Chemistry, 13, 6802–6814.
Gayathri, K., Kumar, A. M., Yerukalapudi, C. S., Natesh, N. S., Rajender, B., & Ramyakrishna, P. (2021). Combination of ZnO nanoparticle with marine sponge derived dipeptide for enhanced anticancer efficacy in liver cancer cells and their toxicity evaluation on embryonic Zebrafish. Journal of Current Analytical Chemistry, 17(5), 677–688.
George, A., Raj, M. A., D., Venci, X., Dhayal Raj, A., Albert Irudayaraj, A., et al. (2022). Photocatalytic effect of CuO nanoparticles flower like 3D nanostructures under visible light irradiation with the degradation of methylene blue (MB) dye for environmental application. Environmental Research, 203, 111880.
Ghitman, J., Biru, E. I., Stan, R., & Iovu, H. (2020). Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Material and Design, 193, 108805.
Balaji, P., Paramasivam, S., Palanisamy, G., Senthilkumar, N., Lalitha, G., Pazhanivel, T., (2023) Photocatalytic Degradation of Tetracycline Contaminated Wastewater over Bi2S3/BiWO6/rGO Ternary Nanocomposite Under Visible Light Irradiation, Journal of the Taiwan Institute of Chemical Engineers, 105249
Govindasamy, G. H., Mydin, R. B. S. M. N. S., Sreekantan, S., & Harun, N. H. (2021a). Compositions and antimicrobial properties of binary ZnO–CuO nanocomposite encasulated calcium and carbon from calotropis gigantea trageted for skin pathogens. Science Reports, 11, 99.
Govindasamy, G. A., Mydin, R. B. S. N., Sreekantan, S., & Harun, N. H. (2021b). Compositions and antimicrobial properties of binary ZnO–CuO nanocomposites encapsulated calcium and carbon from Calotropis gigantea targeted for skin pathogens. National Library of Medicine. https://doi.org/10.1038/s41598-020-79547-w
Jan, T., Azmat, S., Mansoor, Q., Waqas, H. M., et al. (2019). Superior antibacterial activity of ZnO–CuO nanocomposite synthesized by a chemical co-precipitation approach. Microbial Pathogenesis, 134, 103579.
Jelani, M., Li, Z., Shen, Z., et al. (2018). Thermo mechanical response of aluminum alloys under the combined action of tensile loading and laser irradiations. Chinese Physics B, 27(3), 37901–037901.
Jerry, O., Adeyemi, Damian, C., & Onwudiwe., Adebola, O., Oyedeji. (2022). Biogenic synthesis of CuO, ZnO, and CuO–ZnO nanoparticles using leaf extracts of dovyaliscaffra and their biological properties. Molecules, 27(10), 3206.
Kamburova, K., Boshkova, N., Boshkov, N., & Radeva, T. (2021). Composite coatings with polymeric modified ZnO nanoparticles and nanocontainers with inhibitor for corrosion protection of low carbon steel. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 609, 125741.
Kaviyarasu, K., Maria Magdalane, C., Kanimozhi, K., et al. (2017). Elucidation of photocatalysis, photoluminescence and antibacterial studies of ZnO thin films by spin coating method. Journal of Photochemistry and Photobiology B: Biology, 173, 466–475.
Klinbumrung, A., Thongtem, T., & Thongtem, S. (2014). Characterization and gas sensing properties of CuO synthesized by DC directly applying voltage. Applied Surface Science, 313, 640–646.
Koshimitsu, Y., Inoue, G., Sayed, M., Saad, A., Ikeda, M., & Tagami, J. (2020). Transverse micro radiography analysis of the effect of experimental calcium-containing primer system on demineralized enamel. Crystals, 10, 1087.
Lakshmeesha, T. R., Sateesh, M. K., Daruka, B., Prasad, C., Sharma, S. C., et al. (2014). Reactivity of crystalline ZnO super structure against fungi and bacterial pathogens: Synthesized using Nerium oleander leaf extract. Crystal Growth Design, 14, 4068–4079.
Li, B., & Wang, Y. (2010). Facile synthesis and photocatalytic activity of ZnO–CuOnanocomposite. Superlattices and Microstructures, 47, 615–623.
Liang, F. X., Gao, Y., Xie, C., Tong, X. W., Li, Z. J., & Luo, L. B. (2018). Recent advances in the fabrication of graphene ZnO heterojunction for optoelectronic device applications. Journal of Materials Chemistry C, 6, 3815.
Lu, Y. J., Liu, C. F., Hu, C. C., Kuo, J. H., & Rajender, B. (2017). Fabrication and characterization of ZnOnanowires array electrodes with high photocurrent densities: Effects of the seed layer calcinations time. Materials Chemistry and Physics, 189, 56–63.
Lu, Y., Liu, L., Zhang, Z., Zhang, W., Wang, A., Tian, F., Zhao, W., & Yan, J. (2021). Green synthesis of CuO nanoparticles loaded ZnO nanowires arrays with enhanced photocatalytic activity. Materials Chemistry and Physics, 267, 124703.
Mahmoud, N., Mohaddeseh, S., Siavash, I., & Verma, R. S. (2021). Green-synthesized nanocatalysts and nanomaterials for water treatment: Current challenges and future perspectives. Journal of Hazardous Materials, 401, 123401.
Maria Magdalane, C., Kaviyarasu, K., Judith Vijaya, J., Siddhardha, B., & Jeyaraj, B. (2016). Photocatalytic activity of binary metal oxide nanocomposite of CeO2/CdOnanospheres: investigation of optical and antimicrobial activity. Journal of Photochemistry and Photobiology b: Biology, 163, 77–86.
Mohammadi-Aloucheh, R., Habibi-Yangjeh, A., Bayram, A., Latifi-navid, S., & Asadi, A. (2018). Green synthesis of ZnO and ZnO/CuOnanocomposite in Menthalongifolialeaf extract: Characterization and their application as anti-bacterial agents. Journal of Materials in Elextronics, 29, 13596–13605.
Munawar, T., Yasmeen, S., Hussain, F., Mahmood, K., Hussain, A., Asghar, M., & Iqbal, F. (2020). Synthesis of novel hetero structured ZnO-CdO-CuOnanocomposite: characterization and enhanced sunlight driven photocatalytic activity. Materials Chemistry and Physics, 249, 122983.
Murugan, K., Dinesh, D., Kavithaa, K., et al. (2016). Hydrothermal synthesis of titanium dioxidde nanoparticles mosquitocidal potential and anticancer activity on human brest cancer cells (MCF-7). Parasitology Research., 115, 1085–1096.
Ngullie, R. C., Bhuvaneswari, K., Shanmugam, P., Boonyuen, S., Smith, S. M., & Sathishkumar, M. (2022). Magnetically recoverable biomass-derived carbon-AerogelSupportedZnO (ZnO/MNC) composites for the photodegradation of methylene blue. Catalysts, 12(9), 1–14.
Nithiyadevi, K., & Ravichandran, K. (2017). Enhanced visible light responsive photocatalysisby ZnO:Mg/RGO nanocomposite. Journal of Materials Science: Materials in Electronics, 28, 10929–10939.
Padmavathy, N., & Vijayaraghavan, R. (2008). Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Science and Technology of Advanced Materials, 9, 35004.
Pakzad, K., Alinezhad, H., & Nasrollahzadeh, M. (2019). Green synthesis of Ni@Fe3O4 and CuOnanoparticles using Euphorbia maculata extract as photocatalysts for the degradation of organic pollutants under UV-irradiation. Ceramics International, 45, 17173.
Preethi, S., Abama, K., Nithyasri, M., et al. (2020). Synthesis and characterization of chitosan/zinc oxide nanocomposite for antibacterial activity onto cotton fabrics and dye degradation applications. International Journal of Biomedical Macromolecules, 164, 2779–2787.
Qamar, M. T., Aslam, M., Ismail, I. M., Salah, N., & Hameed, N. (2015). Synthesis, characterization, and sunlight mediated photocatalytic activity of CuO-coated ZnO for the removal of nitrophenols. ACS Applied Materials and Interfaces, 7(16), 8757–8769.
Rajender, B., Shanmugam, P., Srivatsava, R. K., Tabassum, N., Pothu, R., Naik, R., Saran, A., Viswanadham, B., Radwan, A. B., & Qahtani, N. A. (2023). Catalytic valorisation of biomass-derived levulinic acid to biofuel additive γ-Valerolactone: Influence of copper loading on silica support. Reactions, 4(3), 465–477.
Rajith Kumar, C. R., Betageri, V. S., Nagaraju, G., Pujar, G. H., Onkarppa, H. S., & Latha, M. S. (2020). One pot green synthesis of ZnO–CuO nanocomposite and their enhanced photocatalytic and antibacterial activity. Advances in Natural Sciences Nanoscience and Nanotechnology, 11, 015009.
Rajivgandhi, G. N., Ramachandran, G., Alharbi, N. S., Kadaikunnan, S., Khaleed, J. M., Manokaran, N., & Li, W. J. (2021). Substantial effect of Cr doping on the antimicrobial activity of ZnO nanoparticles prepared by ultrasonication process. Materials Science and Engineering B: Solid State and Materials for Advanced Technology, 263, 114817.
Ramyakrishna, P., Challa, P., Rajesh, R., Boddula, R., Balaga, R., Balla, P., Perugopu, V., Radwan, A. B., Abdullah, A. M., & Qahtani, N. A. (2022). Vapour-phase selective hydrogenation of γ-Valerolactone to 2-methyltetrahydrofuran biofuel over silica-supported copper catalysts. Nanomaterials, 12(19), 3414.
Rangayasami, A., Kannan, K., Subban, M., & Radhika, D. (2021). Review of photocatalytic and antimicrobial properties of metal oxide nanoparticles. Solid State Physical and Chemistry, 22, 5–15.
Sakib, A. A. M., Masum, S. S. M., Hoinis, J., Isalm, R., & Molla, A. I. (2019). Synthesis of CuO/ZnOnanocomposite and their application in photodegradation of toxic textile dye. Journal of Composite Science, 3, 91.
Saleh, T. A., & Gupta, V. K. (2011). Functionalization of tungsten oxide into MWCNT and its application for sunlight induced degradation of rhodamine B. Journal of Colloid and Interface Science, 362, 337–344.
Sa-nguanprang, S., Phuruangrat, A., Karthik, K., Thongtem, S., & Thongtem, T. (2020). Tartaric assisted precipitation of visible light driven Ce-doped ZnO nanoparticles used for photodegradation of methylene blue. Journal of the Australian Ceramic Society, 56, 1029–1041.
Saravanan, R., Joicy, S., Gupta, V. K., Narayanane, V., & Stephen, A. (2013). The photocatalytic activity of ZnO prepared by simple thermal decomposition method at various temperatures. Journal of Molecular Liquids, 33, 394–401.
Satdeve, N., Ugwekar, R., & Bhanvase, B. (2019). Ultrasound assisted preparation and characterization of Ag supported on ZnO nanoparticles for visible light degradation of methylene blue dye. Journal of Molecular Liquids, 291, 111313.
Shanmugam, P., Ngullie, R. C., Smith, S. M., Boonyuen, S., Rajender, B., & Ramyakrishna, P. (2023). Visible-light induced photocatalytic removal of methylene blue dye by copper oxide decorated zinc oxide nanorods. Materials Science for Energy Technologies, 6, 359–367.
Shanmugam, P., Parasuraman, B., Boonyuen, S., Thangavelu, P., AlSalhi, M. S. T., Zheng, A. L., & Viji, A. (2024). Hydrothermal synthesis and photocatalytic application of ZnS-Ag composites based on biomass-derived carbon aerogel for the visible light degradation of methylene blue. Environmental Geochemistry and Health, 46, 92.
Subhan, M. A., Uddin, N., Sarker, P., Nakata, H., & Makioka, R. (2015). Synthesis, characterization, low temperature solid PL and photocatalytic activities of Ag2O.CeO2.ZnOnanocomposite. SpectrochimicaActa Part a: Molecular and Biomolecular Spectroscopy, 151, 56–63.
Sundrarajan, M., Ambika, S., & Bharathi, K. (2015). Plant extract mediated synthesis of ZnOnanoparticles using Pongamiapinnata and their activity against pathogenic bacteria. Advanced Powder Technology, 26, 1294–1299.
TaghaviFardood, S., Forootan, R., Moradnia, F., Afshari, Z., & Ramazani, A. (2020). Green synthesis, characterization, and photocatalytic activity of cobalt chromite spinel nanoparticles. Material Research Express, 7, 15086.
Taufique, M. F. N., Haque, A., Karnati, P., & Ghosh, K. (2018). ZnO–CuO nanocompostie with improved photocatalytic activity for environmental and energy applications. Journal of Electronic Materials, 47, 6731–6745.
Thomas, D., Rakesh, K. E., Sadasivuni, K. K., et al. (2022). Photosensitivity and photocatalytic activity of ZnO thin films annealed in different environmental conditions. Journal of Electronic Materials, 51, 3992–4004. https://doi.org/10.1007/s11664-022-09651-2
Ullah, H., Mushtaq, L., Ullah, Z., Fazal, A., & Muhammad Khan, A. (2020). Effect of vegetable waste water on microstructure, morphology and photocatalytic efficiency of ZnO–CuOnanocomposite. Inorganic and Nano Metal Chemistry, 51, 963–975.
Usan, P. S. P., Paramasivam, S., Supakorn, B., Lakshmi Prabha, C., Ramyakrishna, P., Rajender, B., Ahmed, B. R., & Al-Q, N. (2024). Non-Covalent functionalization of surfactant-assisted graphene oxide with silver nanocomposites for highly efficient photocatalysis and anti-biofilm applications. Materials Science for Energy and Technology, 7, 205–215.
Wang, S., Tang, S., Yang, H., Yang, F., Yu, C., Gao, H., Fan, G. L., Sun, G., Yi, Z., & Li, D. (2022). A Novel heterojunction ZnO/CuO piezoelectric catalytic activity for efficient degradation of methylene blue. Journal of Materials Science: Materials in Electronics, 33, 7172–7190.
Yatskiv, R., Tiagulskyi, S., Grym, J., Vanis, J., Basinova, N., Horak, P., Torrisi, A., Ceccio, G., Vacik, J., & Vrnata, M. (2019). Optical and electrical characterization of CuO/ZnOheterojunctions. Thin Solid Films, 693, 137656.
Yulizar, Y., Bakri, R., Apriandanu, D. O. B., & Hidayat, T. (2018). ZnO/CuO nanocomposite prepared in one-pot green synthesis using seed bark extract of Theobroma cacao. Nature Structure and Nano Objects, 16, 300–305.
Zhang, Q., Zhang, K., Xu, D., et al. (2014). CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Materials Science and Engineering, 60, 208–337.
Acknowledgements
The author (R.A.) thanks the Department of Botany, Padmavani Arts and Science College for Women, Salem.This work was supported by Qatar University through a National Capacity Building Program Grant (NCBP) [QUCPCAM-22/24-463]. The project was also supported by Researchers Supporting Project number (RSPD2024R675), King Saud University, Riyadh, Saudi Arabia. Statements made herein are solely the responsibility of the authors.
Funding
Open Access funding provided by the Qatar National Library. Open Access funding provided by the Qatar National Library.
Author information
Authors and Affiliations
Contributions
Rangayasami Aswini: Conceptualization, Investigation, Writing—original draft. Kannupaiyan Jothimani: Visualization and Investigation. Karthik Kannan and Ramyakrishna Pothu: Writing – review & editing, Methodology and Formal analysis. Paramasivam Shanmugam: Formal analysis. Rajender Boddula: Data curation, Project administration, Writing—review & editing. Govindasami Periyasami: Writing – review & editing. Perumal Karthikeyan: Software. Noora Al-Qahtani: Supervision and Validation, Writing—review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aswini, R., Jothimani, K., Kannan, K. et al. Carica Papaya leaf-infused metal oxide nanocomposite: a green approach towards water treatment and antibacterial applications. Environ Geochem Health 46, 334 (2024). https://doi.org/10.1007/s10653-024-02090-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10653-024-02090-4