Abstract
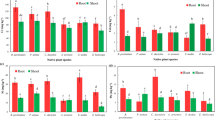
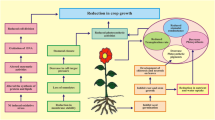
Soils salinization along with heavy metals contamination is among the serious environmental menaces. The present experiment was conducted to study the combined influence of salinity and nickel (Ni) on growth and physiological attributes of quinoa (Chenopodium quinoa Willd.). Thirty-day-old healthy and uniform seedlings of quinoa genotype A7 were exposed to different concentrations of Ni (0, 100, 200, 400 µM), NaCl (0, 150, 300 mM) and their combinations for three weeks. Results indicated that plant growth, pigments and stomatal conductance decreased with increasing Ni concentrations in nutrient solution. Combining lower level of salt (150 mM NaCl) with Ni resulted in improvement in growth and physiological attributes of quinoa. However, the combined application of higher level of salt (300 mM NaCl) with Ni was more detrimental for plant growth and caused more oxidative stress (H2O2 and TBARS) than the alone treatments. The oxidative stress was mitigated by 5.5-fold, 5-fold and 15-fold increase in the activities of SOD, CAT and APX, respectively. The concentration of Na was increased, while K and Ni decreased under the combined treatment of Ni and salinity. Multivariate analysis revealed that a moderate level of salinity had positive effects on growth and Ni phytoremediation potential of quinoa. The higher tolerance index, bioconcentration factor and lower translocation factor depicted that quinoa genotype A7 can be cultivated for phytostabilization of Ni under salinity stress. It was concluded that NaCl salinity level of 150 mM is promising for increasing growth of quinoa on Ni contaminated soils.





Similar content being viewed by others
Availability of data and materials
Data will be available as demanded.
References
Abbas, G., Amjad, M., Saqib, M., Murtaza, B., Naeem, M. A., Shabbir, A., & Murtaza, G. (2021). Soil sodicity is more detrimental than salinity for quinoa (Chenopodium quinoa Willd):A multivariate comparison of physiological, biochemical and nutritional quality attributes. Journal of Agronomy and Crop Science, 207(1), 59–73.
Abbas, G., Chen, Y., Khan, F. Y., Feng, Y., Palta, J. A., & Siddique, K. H. (2018). Salinity and low phosphorus differentially affect shoot and root traits in two wheat cultivars with contrasting tolerance to salt. Agronomy, 8(8), 155.
Abdal, N., Abbas, G., Asad, S. A., Ghfar, A. A., Shah, G. M., Rizwan, M., Ali, S., & Shahbaz, M. (2021). Salinity mitigates cadmium induced phytotoxicity in quinoa (Chenopodium quinoa Willd) by limiting the Cd uptake and improved responses to oxidative stress: implications for phytoremediation. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-01082-y
Adolf, V. I., Jacobsen, S. E., & Shabala, S. (2013). Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environmental and Experimental Botany, 92, 43–54.
Adolf, V. I., Shabala, S., Adolf, V. I., Shabala, S., Andersen, M. N., Razzaghi, F., & Jacobsen, S. E. (2012). Varietal differences of quinoa’s tolerance to saline conditions. Plant and Soil, 357, 117–129.
Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126.
Ain, Q., Akhtar, J., Amjad, M., Haq, M., & Saqib, Z. (2016). Effect of enhanced nickel levels on wheat plant growth and physiology under salt stress. Communications in Soil Science and Plant Analysis, 47, 2538–2546.
Amako, K., Chen, G. X., & Asada, K. (1994). Separate assays specific for ascorbate peroxidase and guaiacol peroxidase and for the oroplastic and cytosolic isoenzymes of ascorbate peroxidase in plants. Plant Cell Physiology, 35, 497–504.
Ameen, N., Amjad, M., Murtaza, B., Abbas, G., Shahid, M., Imran, M., & Niazi, N. K. (2019). Biogeochemical behavior of nickel under different abiotic stresses: toxicity and detoxification mechanisms in plants. Environmental Science and Pollution Research, 26(11), 10496–10514.
Amjad, M., Akhtar, J., Murtaza, B., Abbas, G., & Jawad, H. (2016). Differential accumulation of potassium results in varied salt-tolerance response in tomato (Solanum lycopersicum L.) cultivars. Horticulture Environment and Biotechnology, 57, 248–258.
Amjad, M., Akhtar, S. S., Yang, A., Akhtar, J., & Jacobsen, S. E. (2015). Antioxidative response of quinoa exposed to iso-osmotic, ionic and non-ionic salt stress. Journal of Agronomy and Crop Science, 201, 452–460.
Amjad, M., Ameen, N., Murtaza, B., Abbas, G., Imran, M., Naeem, M. A., Bhutta, W. M., Zakir, A., Masood, N., & Jacobsen, S. E. (2019). A comparative analysis of salinity and nickel tolerance of tomato (Solanum lycopersicum L.). Communications in Soil Science and Plant Analysis, 50(18), 2294–2308.
Amjad, M., Ameen, N., Murtaza, B., Imran, M., Shahid, M., Abbas, G., & Jacobsen, S. E. (2020). Comparative physiological and biochemical evaluation of salt and nickel tolerance mechanisms in two contrasting tomato genotypes. Physiologia Plantarum, 168(1), 27–37.
Brune, A., & Dietz, K. J. (1995). A comparative analysis of element composition of roots and leaves of barley seedlings grown in the presence of toxic cadmium, molybdenum, nickel, and zinc concentrations. Journal of Plant Nutrition, 18, 853–886.
Estefan, G. (2013). Methods of soil, plant, and water analysis. a manual for the West Asia and North Africa Region. In: International Center for Agricultural Research in the Dry Areas (ICARDA), Beirut, Lebanon, 244
Fashola, M. O., Ngole-Jeme, V. M., & Babalola, O. O. (2016). Heavy metal pollution from gold mines: environmental effects and bacterial strategies for resistance. International Journal of Environmental Research and Public Health, 13(11), 1047.
Guarino, F., Ruiz, K. B., Castiglione, S., Cicatelli, A., & Biondi, S. (2020). The combined effect of Cr (III) and NaCl determines changes in metal uptake, nutrient content, and gene expression in quinoa (Chenopodium quinoa Willd.). Ecotoxicology and Environmental Safety, 193, 110–345.
Gupta, A. S., Heinen, J. L., Holaday, A. S., Burke, J. J., & Allen, R. D. (1993). Increased resistance to oxidative stress in transgenic plants that over express oroplastic Cu/Zn superoxide dismutase. Proceedings of National Academy of Sciences, 90, 1629–1633.
Hoagland, D.R., Arnon D.I. (1950). The water-culture method for growing plants without soil, Circular. California Agricultural Experiment Station, 347
Hodges, D. M., DeLong, J. M., Forney, C. F., & Prange, R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta, 207, 604–611.
Hu, Y., Hackl, H., & Schmidhalter, U. (2017). Comparative performance of spectral and thermographic properties of plants and physiological traits for phenotyping salinity tolerance of wheat cultivars under simulated field conditions. Functional Plant Biology, 44, 134–142.
Iftikhar, A., Abbas, G., Saqib, M., Shabbir, A., Amjad, M., Shahid, M., Ahmad, I., Iqbal, S., & Qaisrani, S. A. (2021). Salinity modulates lead (Pb) tolerance and phytoremediation potential of quinoa: a multivariate comparison of physiological and biochemical attributes. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-00937-8
Islam, E., Liu, D., Li, T., Yang, X., Jin, X., Mahmood, Q., Tian, S., & Li, J. (2008). Effect of Pb toxicity on leaf growth, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. Journal of Hazardous Materials, 154, 914–926.
Kabata-Pendias, A., & Pendias, H. (2000). Trace elements in soils and plants. CRC Press.
Kotapati, K. V., Palaka, B. K., & Ampasala, D. R. (2017). Alleviation of nickel toxicity in finger millet (Eleusine coracana L.) germinating seedlings by exogenous application of salicylic acid and nitric oxide. Crop Journal, 5(3), 240–250.
Lee, M. C., Park, J. C., Kim, D. H., Kang, S., Shin, K. H., Park, H. G., Han, J., & Lee, J. S. (2017). Interrelationship of salinity shift with oxidative stress and lipid metabolism in the monogonont rotifer Brachionus koreanus. Comparative Biochemistry and Physioogyl- A Molecular and Integrative Physiology, 214, 79–84.
Li, X., Zhang, X., Wang, X., Yang, X., & Cui, Z. (2019). Bioaugmentation-assisted phytoremediation of lead andsalinity co-contaminated soil by Suaeda salsa and Trichoderma asperellum. Chemosphere, 224, 716–725.
Lichtenthaler, H. K. (1987). orophylls and acrotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology, 148, 350–382.
Manousaki, E., & Kalogerakis, N. (2009). Phytoextraction of Pb and Cd by the Mediterranean saltbush (Atriplex halimus L.): metal uptake in relation to salinity. Environmental Science and Pollution Research, 16(7), 844–854.
Marrugo, N. J., Durango, H. J., Pinedo, H. J., Olivero, V. J., & Diez, S. (2015). Phytoremediation of mercury-contaminated soils byJatropha curcas. Chemosphere, 127, 58–63.
Marschner, H. (1995). Mineral nutrition of higher plants. 2nd Ed, Academic Press London.
Marschner, P. (2012). Rhizosphere biology. In Marschner's mineral nutrition of higher plants. 369–388. Academic Press.
Munns, R. (2005). Genes and salt tolerance: bringing them together. New Phytologist, 167, 645–663.
Munns, R., & Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59, 651–681.
Natasha, Hasnain, Shahid, Muhammad, Sardar, Aneeza, Sana, Anwar, Sajid, Khalid, Bilal, Hussain, Haidar, Ali, & Muhammad, Shah. (2020). Effect of co-application of wastewater and freshwater on the physiological properties and trace element content in Raphanus sativus: soil contamination and human health. Environmental Geochemistry and Health., 43(2393), 2406.
Naveed, M., Ditta, A., Ahmad, M., Mustafa, A., Ahmad, Z., Conde-Cid, M., Tahir, S., Shah, S. A. A., Abrar, M. M., & Fahad, S. (2021). Processed animal manure improves morpho-physiological and biochemical characteristics of Brassica napus L. under nickel and salinity stress. Environmental Science and Pollution Research, 28, 45629–45645.
Nawaz, M. F., Gul, S., Tanvir, M. A., Akhtar, J., Chaudary, S., & Ahmad, I. (2016). Influence of NaCl-salinity on Pb-uptake behavior and growth of River red gum tree (Eucalyptus camaldulensis Dehnh.). Turkish Journal of Agriculture and Forest, 40, 425–432.
Nikalje, G. C., & Suprasanna, P. (2018). Coping with metal toxicity-cues from halophytes. Frontiers of Plant Science, 9, 777.
Parlak, K. U. (2016). Effect of nickel on growth and biochemical characteristics of wheat (Triticum aestivum L.) seedlings. NJAS: Wageningen. Journal of Life Sciences, 76(1), 1–5.
Parvez, S., Abbas, G., Shahid, M., Amjad, M., Hussain, M., Asad, S. A., & Naeem, M. A. (2020). Effect of salinity on physiological, biochemical and photostabilizing attributes of two genotypes of quinoa (Chenopodium quinoa Willd.) exposed to arsenic stress. Ecotoxicology and Environmental Safety, 187, 109814.
Patterson, J. H., Newbigin, E. D., Tester, M., Bacic, A., & Roessner, U. (2009). Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. Journal of Experimental Botany, 60(14), 4089–4103.
Qadir, M., Quillerou, E., Nangia, V., Murtaza, G., Singh, M., Thomas, R. J., & Noble, A. D. (2014). Economics of salt induced land degradation and restoration. Natural Resources Forum, 38, 282–295.
Rangani, J., Parida, A. K., Panda, A., & Kumari, A. (2016). Coordinated changes in antioxidative enzymes protect the photosynthetic machinery from salinity induced oxidative damage and confer salt tolerance in an extreme halophyte (Salvadora persica L.). Frontiers of Plant Science, 10, 7–50.
Razzaghi, F., Jacobsen, S. E., Jensen, C. R., & Andersen, M. N. (2015). Ionic and photosynthetic homeostasis in quinoa challenged by salinity and drought–mechanisms of tolerance. Functional Plant Biology, 42(2), 136–148.
Rehman, S., Abbas, G., Shahid, M., Saqib, M., Farooq, A. B. U., Hussain, M., Murtaza, B., Amjad, M., Naeem, M. A., & Farooq, A. (2019). Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stress: Implications for phytoremediation. Ecotoxicology and Environmental Safety, 171, 146–153.
Riaz, F., Abbas, G., Saqib, M., Amjad, M., Farooq, A., Ahmad, S., & Ahmad, N. (2020). Comparative effect of salinity on growth, ionic and physiological attributes of two quinoa genotypes. Pakistan Journal of Agricultural Sciences, 57, 115–122.
Ruiz, K. B., Biondi, S., Martinez, E. A., Orsini, F., Antognoni, F., & Jacobsen, S. E. (2015). Quinoa a model crop for understanding salt tolerance mechanisms in halophytes. International Journal of Plant Biology, 150, 357–371.
Shabala, S. (2013). Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany, 112(7), 1209–1221.
Shabbir, A., Saqib, M., Murtaza, G., Abbas, G., Imran, M., Rizwan, M., & Javeed, H. M. R. (2021). Biochar mitigates arsenic-induced human health risks and phytotoxicity in quinoa under saline conditions by modulating ionic and oxidative stress responses. Environmental Pollution, 287, 117348.
Shahbaz, A. K., Lewinska, K., Iqbal, J., Ali, Q., Iqbal, M., Abbas, F., & Ramzani, P. M. A. (2018). Improvement in productivity, nutritional quality, and antioxidative defense mechanisms of sunflower (Helianthus annuus L.) and maize (Zea mays L.) in nickel contaminated soil amended with different biochar and zeolite ratios. Journal of Environmental Management, 218, 256–270.
Steel, R., Torrie, J., & Dickey, D. (1997). Principles and Procedures of Statistics: A Biometrical Approach (3rd ed.). McGraw-Hill.
Stetsenko, L. A., Kozhevnikova, A. D., & Kartashov, A. V. (2017). Salinity attenuates nickel accumulating capacity of Atropa belladonna L plants. Russian Journal of Plant Physiology, 64(4), 486–496.
Van-Oosten, M. J., & Maggio, A. (2015). Functional biology of halophytes in the phytoremediation of heavy metal contaminated soils. Environmental and Experimental Botany, 111, 135–146.
Yilmaz, D. D. (2007). Effects of salinity on growth and nickel accumulation capacity of Lemna gibba (Lemnaceae). Journal of Hazardous Materials, 147(1–2), 74–77.
Yusuf, M., Fariduddin, Q., Hayat, S., & Ahmad, A. (2011a). Nickel: an overview of uptake, essentiality and toxicity in plants. Bulletin of Environmental Contamination and Toxicology, 86, 1–17.
Yusuf, M., Fariduddin, Q., Hayat, S., Hasan, S. A., & Ahmad, A. (2011b). Protective response of 28-homobrassinolide in cultivars of Triticum aestivum with different levels of nickel. Archives of Environmental Contamination and Toxicology, 60, 68–76.
Zurayk, R. A., Khoury, N. F., Talhouk, S. N., & Baalbaki, R. Z. (2001). Salinity-heavy metal interactions in four salt-tolerant plant species. Journal of Plant Nutrition, 24(11), 1773–1786.
Acknowledgements
The authors are highly thankful to the COMSATS University Islamabad, Vehari Campus for providing research facilities. Authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP-2021/194), King Saud University, Riyadh, Saudi Arabia.
Funding
The authors are greatly thankful to COMSATS University Islamabad, Vehari Campus for the support. The authors are also grateful to the Researchers Supporting Project number (RSP-2021/194), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
G.A. and M.A.N. conceived the research idea. G.A. and M.Z.M analyzed the data and wrote the manuscript. N.N. and M.H. accomplished the experimentation and plant analyses. R.S., S.A. and M.H.S. reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Naheed, N., Abbas, G., Naeem, M.A. et al. Nickel tolerance and phytoremediation potential of quinoa are modulated under salinity: multivariate comparison of physiological and biochemical attributes. Environ Geochem Health 44, 1409–1424 (2022). https://doi.org/10.1007/s10653-021-01165-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-01165-w