Abstract
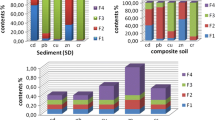
A new index is proposed to determine the affinity of heavy metals (HM) to their carrier phases (AHM-fraction), which, in contrast to the traditional index CHM = 100 CHM-fraction/CHM-soil, considers the sum of all metals in the fraction as a share of the bulk content of all HM in the soil. The metal has affinity for the given phase if AHM-fraction > 1; vice versa, the affinity is absent if AHM-fraction < 1. Comparison of the affinity series of metals for a certain phase based on two indices revealed their discrepancy in most cases. The new index can take into consideration the discrepancy in affinity of the given metal for phases extracted by different strength reagents. The effect of the new indicator was tested on several contaminated soils: Haplic Chernozem, Stagnic Phaeozems, and Calcaric Fluvic Arenosol, as well as on two Spolic Technosols. Compared with the index CHM, the results of the new analysis of contaminated soils with the ATM fraction demonstrated that the Zn content in Calcaric Fluvic Arenosol is decreased considerably due to its low buffer capacity. Since the content of organic matter in Calcaric Fluvic Arenosol is insignificant, only organophile elements, such as Cu and Pb, can make up complexes with organic ligands, in contrast to the fixation of Ni and Mn by organic matter in Chernozems. Due to the low buffering capacity of Calcaric Fluvic Arenosol, the mobile forms of Cd and Zn increased, and these forms of Cr decreased. Therefore, the low buffering soil cannot fix Cd and Zn. Increase in contamination in Spolic Technosols (approximate permissible concentration, APC > 5) as compared to the index CHM, the value of the AHM-fraction of metals in the residue (except for cadmium) increased. In addition, the share of Pb and Cu increases in the organic matter. Thus, the use of a new indicator—the affinity of heavy metals to the carrier phases showed their advantage over the traditional index CHM.
Similar content being viewed by others
References
Acosta, J. A., Gabarron, M., Faz, A., Martınez-Martınez, S., Zornoza, R., & Arocena, J. M. (2015). Influence of population density on the concentration and speciation of metals in the soil and street dust from urban areas. Chemosphere, 134, 328–337. https://doi.org/10.1016/j.chemosphere.2015.04.038
Bacon, J. R., & Davidson, C. M. (2008). Is there a future for sequential chemical extraction? The Analyst, 133, 25–46. https://doi.org/10.1039/b711896a
Bauer, T. V., Linnik, V. G., Minkina, T. M., Mandzhieva, S. S., & Nevidomskaya, D. G. (2018). Ecological-geochemical studies of technogenic soils in the flood plain landscapes of the Seversky Donets. Lower Don Basin. Geochemistry International, 56(10), 992–1002. https://doi.org/10.1134/S001670291810004X
Bol’shakov V. A., Belobrov, V. P., Shishov, L. L., (2004). Glossary. Terms, their short definitions, reference materials on soil ecology, geography and soil classification. Moscow: Dokuchaev soil science institute, (p. 138) (in Russian).
Ecological bulletin of the don: On the state of the environment and natural resources of the Rostov region in 2019. (2020). Rostov-on-Don (in Russian).
Evans, Z. C., Ryswyk, H. V., Huertos, M. L., & Srebotnjak, T. (2019). Robust spatial analysis of sequestered metals in a Southern California Bioswale. Science of The Total Environment, 650(1), 155–162. https://doi.org/10.1016/j.scitotenv.2018.08.441
Fedotov, P. S., Rogova, O. B., Dzhenloda, RKh., & Karandashov, V. K. (2019). Metal-organic complexes in soils as a major sink for rare earth elements. Environmental Chemistry, 16(5), 323–332. https://doi.org/10.1071/EN18275
Ghosh, P., Samanta, A. N., & Ray, S. (2011). Reduction of COD and removal of Zn2+ from rayon industry wastewater by combined electro-Fenton treatment and chemical precipitation. Desalination, 266(1–3), 213–217. https://doi.org/10.1016/j.desal.2010.08.029
HN 2.1.7.2511-09. (2009). Approxible permissible concentrations (APC) of chemical matters in soil. Moscow: Federal center for hygiene and epidemiology of rospotrebnadzor (in Russian).
Hasan, M., Kausar, D., Akhter, G., & Shah, M. H. (2018). Evaluation of the mobility and pollution index of selected essential/toxic metals in paddy soil by sequential extraction method. Ecotoxicology and Environmental Safety, 147, 283–291. https://doi.org/10.1016/j.ecoenv.2017.08.054
He, Q., Ren, Y., Mohamed, I., Ali, M., Hassan, W., & Zeng, F. (2013). Assessment of trace and heavy metal distribution by four sequential extraction procedures in a contaminated soil. Soil and Water Research, 8, 71–76. https://doi.org/10.17221/20/2012-swr
Huang, Y., Chen, Q., Deng, M., Japenga, J., Li, T., Yang, X., & He, Z. (2018). Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. Journal of Environmental Management, 207, 159–168. https://doi.org/10.1016/j.jenvman.2017.10.072
ISO 10693 (1995). Soil quality—Determination of carbonate content—volumetric method.
ISO 14235 (1998). Soil quality—Determination of organic carbon by sulfochromic oxidation.
ISO 13317-2 (2001). Determination of particle size distribution by gravitational liquid sedimentation methods—Part 2: Fixed pipette method.
ISO 10390 (2005). Soil quality—Determination of pH.
ISO NF EN 23470 (2011). Soil quality—Determination of effective cation exchange capacity (CEC) and exchangeable cations.
IUSS Working Group WRB (2015) World reference base for soil resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. World soil resources reports, FAO, Rome, No. 106.
Kabata-Pendias, A. (2011). Trace elements in soils and plants N.Y. CRC Press. London: Boca Raton, (p. 450).
Karim, Z., Qureshi, B. A., Mumtaz, M., & Qureshi, S. (2014). Heavy metal content in urban soils as an indicator of anthropogenic and natural influences on landscape of Karachi—A multivariate spatio-temporal analysis. Ecological Indicators, 42, 20–31. https://doi.org/10.1016/j.ecolind.2013.07.020
Kartal, S., Aydın, Z., & Tokalioglu, S. (2006). Fractionation of metals in street sediment samples by using the BCR sequential extraction procedure and multivariate statistical elucidation of the data. Journal of Hazardous Materials, 132, 80–89. https://doi.org/10.1016/j.jhazmat.2005.11.091
Ladonin, D.V., Forms of heavy metals compounds in technogenically contaminated soils. Dr. of biological sciences thesis abstract, Moscow, (p. 42) (in Russian).
Land, M., Ohlander, B., Ingri, J., & Thunberg, J. (1999). Soil speciation and fractionation of rare earth elements in a spodosol profile from northern Sweden as revealed by sequential extraction. Chemical Geology, 160, 121–138. https://doi.org/10.1016/S0009-2541(99)00064-9
Linnik, V. G., Minkina, T. M., Bauer, T. V., Saveliev, A. A., & Mandzhieva, S. S. (2020). Geochemical assessment and spatial analysis of heavy metals pollution around coal-fired power station. Environmental Geochemistry and Health, 42(12), 4087–4100. https://doi.org/10.1007/s10653-019-00361-z
Manceau, A., Boisset, M. C., Sarret, G., Hazemann, J. L., Mench, M., Cambier, P., & Prost, R. (1996). Direct determination of lead speciation in contaminated soils by EXAFS spectroscopy. Environmental Science & Technology, 30, 1540–1552
Manceau, A., Marcus, M. A., & Tamura, N. (2002). Quantitative speciation of heavy metals in soils and sediments by synchrotron X-ray techniques. Reviews in Mineralogy and Geochemistry, 49(1), 341–428. https://doi.org/10.2138/gsrmg.49.1.341
Minkina, T. M., Motuzova, G. V., Nazarenko, O. G., Kryshchenko, V. S., & Mandzhieva, S. S. (2008). Forms of heavy metal compounds in soils of the steppe zone. Eurasian Soil Science, 41(7), 708–716. https://doi.org/10.1134/S1064229308070053
Minkina, T. M., Nazarenko, O. G., Motuzova, G. V., & Mandzhieva, S. S. (2009). Group composition of heavy metal compounds in the soils contaminated by emissions from the Novocherkassk power station. Eurasian Soil Science, 42(13), 1533–1542. https://doi.org/10.1134/S1064229309130158
Morin, G., Ostergren, J. D., Juillot, F., Ildefonse, P., Calas, G., & Brown, J. E. (1999). XAFS determination of the chemical form of lead in smelter-contaminated soils and mine tailings: Importance of adsorption process. American Mineralogist, 84, 420–434. https://doi.org/10.2138/am-1999-0327
Nevidomskaya, D. G., Minkina, T. M., Soldatov, A. V., Bauer, T. V., Shuvaeva, V. A., Zubavichus, Y. V., Trigub, A. L., Mandzhieva, S. S., Dorovatovskii, P. V., & Popov, Y. V. (2020). Speciation of Zn and Cu in Technosol and evaluation of a sequential extraction procedure using XAS, XRD and SEM–EDX analyses. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-020-00693-1
Orecchio, S., & Polizzotto, G. (2013). Fractionation of mercury in sediments during draining of Augusta (Italy) coastal area by modified Tessier method. Microchemical Journal, 110, 452–457. https://doi.org/10.1016/j.microc.2013.05.015
Ostergren, J. D., Brown, G. E., Parks, G. A., & Tingle, T. N. (1999). Quantitative speciation of lead in selected mine tailing from Leadvill, CO. Environmental Science & Technology, 33(10), 1627–1636. https://doi.org/10.1021/es980660s
Privalenko, V. V., & Cherkashina, I. F. (2012). Rekul’tivacija shlamonakopitelej himicheskih zavodov v Rostovskoj oblasti [Recultivation of Rostov region chemical industry waste fields]. In T. S. Chibrik (Ed.), Biologicheskaja rekul’tivacija i monitoring narushennyh zemel’ [Biological recultivation and monitoring of disturbed lands]. (pp. 205–209). Publishing House of the Ural University.
Privalenko, V. V., Mazurenko, V. T., Panaskov, V. I., Moshkin, V. M., Mukhin, N. V., & Senin, B. K. (2000). Ecological problems in the city of Kamensk-Shakhtinsk. Tsvetnaya pechat’.
Reimann, C., & de Caritat, P. (1998). Chemical elements in the environment—factsheets for the geochemist and environmental scientist. Springer-Verlag.
Rosado, D., Usero, J., & Morillo, J. (2016). Ability of 3 extraction methods (BCR, Tessier and protease K) to estimate bioavailable metals in sediments from Huelva estuary (Southwestern Spain). Marine Pollution Bulletin, 102, 65–71. https://doi.org/10.1016/j.marpolbul.2015.11.057
Silveira, M. L., Alleoni, L. R. F., O’Connor, G. A., & Chang, A. C. (2006). Heavy metal sequential extraction methods—A modification for tropical soils. Chemosphere, 64, 1929–1938. https://doi.org/10.1016/j.chemosphere.2006.01.018
Strawn, D. G., & Baker, L. L. (2008). Speciation of Cu in a contaminated agricultural soil measured by XAFS, μ-XAFS, and μ-XRF. Environmental Science and Technology, 42(1), 37–42. https://doi.org/10.1021/es071605z
Tessier, A., Campbell, P. G. O., & Bisson, M. (1979). Sequential extraction procedure for the speciation of the particulate trace metals. Analytical Chemistry, 51, 844–851
Vodyanitskii, Yu. N. (2006). Methods of sequential extraction of heavy metals from soils: New approaches and the mineralogical control (a review). Eurasian Soil Science, 39(10), 1074–1083. https://doi.org/10.1134/S106422930610005X
Wang, Yu., Xu, W., Li, J., Song, Y., Hua, M., Li, W., Wen, Yu., Li, T., & He, X. (2021). Assessing the fractionation and bioavailability of heavy metals in soil–rice system and the associated health risk. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-00876-4
Zhao, X., Huang, J., Lu, J., & Sun, Yu. (2019). Study on the influence of soil microbial community on the long-term heavy metal pollution of different land use types and depth layers in mine. Ecotoxicology and Environmental Safety, 170, 218–226. https://doi.org/10.1016/j.ecoenv.2018.11.136
Acknowledgments
The research was financially supported by the Russian Foundation for Basic Research (no. 19-34-60041) and the Grant of the President of the Russian Federation, MK-6137.2021.1.5.
Funding
The research was financially supported by the Russian Foundation for Basic Research (no. 19-34-60041) and the Grant of the President of the Russian Federation, MK-6137.2021.1.5.
Author information
Authors and Affiliations
Contributions
YNV and TB were responsible for data curation. YNV was responsible for methodology, conceptualization and writing—original draft preparation—review and editing. YNV and TM were responsible for supervision. TM was responsible for writing—review and editing. TB was responsible for investigation, formal analysis and writing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Availability of data and material
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Ethical Approval
Not applicable since the manuscript has not been involved the use of any animal or human data or tissue.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vodyanitskii, Y., Minkina, T. & Bauer, T. Methods to determine the affinity of heavy metals for the chemically extracted carrier phases in soils. Environ Geochem Health 44, 1387–1398 (2022). https://doi.org/10.1007/s10653-021-00955-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-00955-6