Abstract
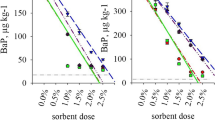
Benzo[a]pyrene (BaP) is a polycyclic aromatic hydrocarbon, highly persistent and toxic and a widespread environmental pollutant. Although various technologies have been developed to remove BaP from the environment, its sorption through solid matrixes has received increasing attention due to cost-effectiveness. The present research compares the adsorption capacity of Haplic Chernozem, granular activated carbon and biochar in relation to BaP from water solution. Laboratory experiments with different initial BaP concentrations in the liquid phase and different ratios of the solid and liquid phases show that Freundlich model describes well the adsorption isotherms of BaP by the soil and both sorbents. Moreover, the BaP isotherm sorption by the Haplic Chernozem is better illustrated by the Freundlich model than the Langmuir equation. The results reveal that the sorption capacity of the carbonaceous adsorbents at a ratio 1:20 (solid to liquid phases) is orders of magnitude higher (13 368 ng mL−1 of activated carbon and 3 578 ng mL−1 of biochar) compared to the soil (57.8 ng mL−1). At the ratio of 0.5:20, the adsorption capacity of the carbonaceous sorbents was 17–45 times higher than that of the soil. This is due to the higher pore volume and specific surface area of the carbonaceous sorbents than soil particles, assessed through scanning electron microscopy. The sorption kinetic of BaP by Chernozem was compared with the adsorption kinetics by the carbonaceous sorbents. Results indicate that the adsorption dynamic involves two steps. The first one is associated with a fast BaP adsorption on the large available surface and inside macro- and meso-pores of the sorbent particles of the granular activated carbon and biochar. Then, the adsorption is followed by a slower process of BaP penetration into the microporous space and/or redistribution into a hydrophobic fraction. The effectiveness of the sorption process depends on both the sorbent properties and the solvent competition. Overall, the granular activated carbon and biochar are highly effective adsorbents for BaP, whereas the Haplic Chernozem has a rather limited capacity to remove BaP from contaminated solutions.





Similar content being viewed by others
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study.
References
Ahmad, M., Rajapaksha, A. U., Lim, J. E., Zhang, M., Bolan, N., Mohan, D., Vithanage, M., Lee, S. S., & Ok, Y. S. (2014). Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere, 99, 19–33. https://doi.org/10.1016/j.chemosphere.2013.10.071
Bao, H., Wang, J., Zhang, H., Li, J., Li, H., & Wu, F. (2020). Effects of biochar and organic substrates on biodegradation of polycyclic aromatic hydrocarbons and microbial community structure in PAHs-contaminated soils. Journal of Hazardous Materials, 385, 121595. https://doi.org/10.1016/j.jhazmat.2019.121595
Battaglia-Brunet, F., Dictor, M.C., Garrido, F., Crouzet, C., Morin, D., Dekeyser, K., Clarens, M., Baranger, P. (2002). An arsenic (III)-oxidizing bacterial population: Selection, characterization and performance in reactors. J. Appl. Microbiol. 93, 656–667. https://doi.org/10.13140/2.1.4202.5923
Beesley, L., Moreno-Jiménez, E., Gomez-Eyles, J. L., Harris, E., Robinson, B., & Sizmur, T. (2011). A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environmental Pollution, 159, 3269–3282. https://doi.org/10.1016/j.envpol.2011.07.023
Brennan, A., Moreno-Jiménez, E., Alburquerque, J. A., Knapp, C. W., & Switzer, C. (2014). Effects of biochar and activated carbon amendment on maize growth and the uptake and measured availability of polycyclic aromatic hydrocarbons (PAHs) and potentially toxic elements (PTEs). Environmental Pollution, 193, 79–87. https://doi.org/10.1016/j.envpol.2014.06.016
Cantrell, K. B., Hunt, P. G., Uchimiya, M., Novak, J. M., & Ro, K. S. (2012). Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresource Technology, 107, 419–428
Chai, Y., Currie, R. J., Davis, J. W., Wilken, M., Martin, G. D., Fishman, V. N., & Ghosh, U. (2012). Effectiveness of activated carbon and biochar in reducing the availability of polychlorinated dibenzo-p dioxins/dibenzofurans in soils. Environmental Science and Technology, 46, 1035–1043. https://doi.org/10.1021/es2029697
Chen, B. L., & Chen, Z. M. (2009). Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere, 76, 127–133. https://doi.org/10.1016/j.chemosphere.2009.02.004
Cheung, W. H., Szeto, Y. S., & McKay, G. (2007). Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresource Technology, 98, 2897–2904. https://doi.org/10.1016/j.biortech.2006.09.045
Chepelev, Nikolai L., et al. (2015). "Neurotoxicity may be an overlooked consequence of benzo[a]pyrene exposure that is relevant to human health risk assessment. Mutation Research/Reviews in Mutation Research. 764: 64–89. https://doi.org/10.1016/j.mrrev.2015.03.001
Cho, H. H., Smith, B. A., Wnuk, J. D., Fairbrother, D. H., & Ball, W. P. (2008). Influence of surface oxides on the adsorption of naphthalene onto multiwalled carbon nanotubes. Environmental Science and Technology, 42, 2899–2905. https://doi.org/10.1021/es702363e
Cornelissen, G., Gustafsson, O., Bucheli, T. D., Jonker, M. T. O., Koelmans, A. A., & van Noort, P. C. M. (2005). Extensive sorption of organic compounds to black carbon, coal and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation and biodegradation. Environmental Science and Technology, 39, 6881–6895. https://doi.org/10.1021/es050191b
Dai, W.-J., Wu, P., Liu, D., Hu, J., Cao, Y., Liu, T.-Z., Okoli, C. P., Wang, B., & Li, L. (2020). Adsorption of polycyclic aromatic hydrocarbons from aqueous solution by organic montmorillonite sodium alginate nanocomposites. Chemosphere, 251, 126074. https://doi.org/10.1016/j.chemosphere.2020.126074
Dai, Y., Niu, J., Yin, L., Xu, J., & Xi, Y. (2011). Sorption of polycyclic aromatic hydrocarbons on electrospun nanofibrous membranes: sorption kinetics and mechanism. Journal of Hazardous Materials, 192, 1409–1417. https://doi.org/10.1016/j.jhazmat.2011.06.055
Denyes, M. J., Rutter, A., & Zeeb, B. A. (2013). In situ application of activated carbon and biochar to PCB-contaminated soil and the effects of mixing regime. Environmental Pollution, 182, 201–208. https://doi.org/10.1016/j.envpol.2013.07.016
Dictor, M. C., Berne, N., Mathieu, O., Moussay, A., & Saada, A. (2003). Influence of ageing of polluted soils on bioavailability of phenanthrene. Oil & Gas Science and Technology, 58, 481–488. https://doi.org/10.2516/ogst:2003031
Eeshwarasinghe, D., Loganathan, P., & Kalaruban, M. (2018). Removing polycyclic aromatic hydrocarbons from water using granular activated carbon: kinetic and equilibrium adsorption studies. Sci. Pollut. Res. https://doi.org/10.1007/s11356-018-1518-0
EFSA (European Food Safety Authority). (2008). Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on polycyclic aromatic hydrocarbons in food. EFSA Journal, 724, 1–114
Fellet, G., Marmiroli, M., & Marchiol, L. (2014). Elements uptake by metal accumulator species grown on mine tailings amended with three types of biochar. Science of the Total Environment, 468–469, 598–608. https://doi.org/10.1016/j.scitotenv.2013.08.072
Funada, M., Nakano, T., & Moriwaki, H. (2018). Removal of polycyclic aromatic hydrocarbons from soil using a composite material containing iron and activated carbon in the freeze-dried calcium alginate matrix: novel soil cleanup technique. Journal of Hazardous Materials, 351, 232–239. https://doi.org/10.1016/j.jhazmat.2018.02.054
Guo, X., Luo, L., Ma, Y., & Zhang, S. (2010). Sorption of polycyclic aromatic hydrocarbons on particulate organic matters. Journal of Hazardous Materials, 173, 130–136. https://doi.org/10.1016/j.jhazmat.2009.08.065
Hale, S. E., Elmquist, M., & Br€andli, R., Hartnik, T., Jakob, L., Henriksen, T., Werner, D., Cornelissen, G. . (2012). Activated carbon amendment to sequester PAHs in contaminated soil: a lysimeter field trial. Chemosphere, 87, 177–184. https://doi.org/10.1016/j.chemosphere.2011.12.015
Hamoudi, S., & Belkacemi, K. (2013). Adsorption of nitrate and phosphate ions from aqueous solutions using organically-functionalized silica materials: kinetic modeling. Fuel, 110, 107–113. https://doi.org/10.1016/j.fuel.2012.09.066
Haro, M., Cabal, B., Parra, J. B., & Ania, C. O. (2011). On the adsorption kinetics and equilibrium of polyaromatic hydrocarbons from aqueous solution. Adsorption Science & Technology, 29, 467–478. https://doi.org/10.1260/0263-6174.29.5.467
Huang, W., & Peng, P.a., Yu, Z. and Fu, J. . (2003). Effects of organic matter heterogeneity on sorption and desorption of organic contaminants by soils and sediments. Applied Geochemistry, 18, 955–972. https://doi.org/10.1016/S0883-2927(02)00205-6
Huggins, T. M., Haeger, A., Biffinger, J. C., & Ren, Z. J. (2016). Granular biochar compared with activated carbon for wastewater treatment and resource recovery. Water Research, 94, 225–232. https://doi.org/10.1016/j.watres.2016.02.059
IARC. (1985). Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume 35: Polycyclic Aromatic Compounds, Part 4, Bitumens, Coal-tars and Derived Products, Shale-oils and Soots; International Agency for Research on Cancer: Lyon, France.
ISO 13877–2005. (2005). Soil Quality-Determination of Polynuclear Aromatic Hydrocarbons - Method Using High-performance Liquid Chromatography.
IUSS Working Group WRB. (2015). World Reference Base for Soil Resources 2014, Update 2015, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106. FAO, Rome.
Jakob, L., Hartnik, T., Henriksen, T., Elmquist, M., Brandli, R. C., Hale, S. E., & Cornelissen, G. (2012). PAH-sequestration capacity of granular and powder activated carbon amendments in soil and their effects on earthworms and plants. Chemosphere, 88, 699–705. https://doi.org/10.1016/j.chemosphere.2012.03.080
Karami, N., Clemente, R., Moreno-Jiménez, E., Lepp, N. W., & Beesley, L. (2011). Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. Journal of Hazardous Materials, 191, 41–48. https://doi.org/10.1016/j.jhazmat.2011.04.025
Ke, Y., Ning, X., Liang, J., Zou, H., Sun, J., Cai, H., Lin, M., Li, R., & Zhang, Y. (2018). Sludge treatment by integrated ultrasound -Fenton process: characterization of sludge organic matter and its impact on PAHs removal. Journal of Hazardous Materials, 343, 191–199. https://doi.org/10.1016/j.jhazmat.2017.09.030
Keiluweit, M., & Kleber, M. (2009). Molecular-level interactions in soils and sediments: the role of aromatic π-systems. Environmental Science and Technology, 43(10), 3421–3429. https://doi.org/10.1021/es8033044
Kołtowski, M., Hilber, I., Bucheli, T. D., Charmas, B., Skubiszewska-Zieba, J., & Oleszczuk, P. (2017). Activated biochars reduce the exposure of polycyclic aromatic hydrocarbons in industrially contaminated soils. Chemical Engineering Journal, 310, 33–40. https://doi.org/10.1016/j.cej.2016.10.065
Kong, Q., Wu, H., Liu, L., Zhang, F., Preis, S., Zhu, S., & Wei, C. (2018). Solubilization of polycyclic aromatic hydrocarbons (PAHs) with phenol in coking wastewater treatment system: Interaction and engineering significance. Science of the Total Environment, 628–629, 467–473. https://doi.org/10.1016/j.scitotenv.2018.02.077
Konstantinova, E., Minkina, T., Sushkova, S., Antonenko, E., & Konstantinov, A. (2020). Levels, sources and toxicity assessment of polycyclic aromatic hydrocarbons in urban topsoils of an intensively developing Western Siberian city. Environmental Geochemistry and Health, 42, 325–341. https://doi.org/10.1007/s10653-019-00357-9
Laird, D. A., Fleming, P., Davis, D. D., Horton, R., Wang, B., & Karlen, D. L. (2010). Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma, 158, 443–449. https://doi.org/10.1016/j.geoderma.2010.05.013
Lamichhane, S., Bal Krishna, K. C., & Sarukkalige, R. (2016). Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: a review. Chemosphere, 148, 336–353. https://doi.org/10.1016/j.chemosphere.2016.01.036
Landers, J., Gor, G. Yu., Neimark, A.V. (2013). Density functional theory methods for characterization of porous materials. Colloids Surf., A. 437, 3–32. https://doi.org/10.1016/j.colsurfa.2013.01.007
Li, F., Chen, J., Hu, X., He, F., Bean, E., Tsang, D. C. W., Ok, Y. S., & Gao, B. (2020). Applications of carbonaceous adsorbents in the remediation of polycyclic aromatic hydrocarbon-contaminated sediments: A review. Journal of Cleaner Production, 255, 120263
Li, Y., Song, N., Yu, Y., Yang, Z., & Shen, Z. (2017). Characteristics of PAHs in street dust of Beijing and the annual wash-off load using an improved load calculation method. Science of the Total Environment, 581–582, 328–336. https://doi.org/10.1016/j.scitotenv.2016.12.133
Liao, W., Ma, Y., Chen, A., & Yang, Y. (2015). Preparation of fatty acids coated Fe3O4 nanoparticles for adsorption and determination of benzo(a)pyrene in environmental water samples. Chemical Engineering Journal, 271, 232–239. https://doi.org/10.1016/j.cej.2015.03.010
Liu, C., Liu, F., Ravnskov, S., Rubæk, G. H., Sun, Z., & Andersen, M. N. (2017). Impact of wood biochar and its interactions with mycorrhizal fungi, phosphorus fertilization and irrigation strategies on potato growth. Journal of Agronomy and Crop Science, 203(2), 131–145. https://doi.org/10.1111/jac.12185
Malakahmad, A., & Ho, L. L. H. (2017). UV/H2O2 oxidation process optimization by response surface methodology for removal of polycyclic aromatic hydrocarbons (PAHs) from water. Desalination and Water Treatment, 65, 408–417
Marchal, G., Smith, K. E. C., Mayer, P., Wollesen de Jonge, L., & Karlson, U. G. (2014). Impact of soil amendments and the plant rhizosphere on PAH behavior in soil. Environmental Pollution, 188, 124–131. https://doi.org/10.1016/j.envpol.2014.02.008
Minkina, T., Sushkova, S., Yadav, B. K., Rajput, V., Mandzhieva, S., & Nazarenko, O. (2019). Accumulation and transformation of benzo[a]pyrene in Haplic Chernozem under artificial contamination. Geochem. Health. https://doi.org/10.1007/s10653-019-00362-y
Minnikova, T. V., Denisova, T. V., Mandzhieva, S. S., Kolesnikov, S. I., Minkina, T. M., Chaplygin, V. A., Burachevskaya, M. V., Sushkova, S. N., & Bauer, T. V. (2017). Assessing the effect of heavy metals from the Novocherkassk power plant emissions on the biological activity of soils in the adjacent areas. Journal of Geochemical Exploration, 174, 70–78. https://doi.org/10.1016/j.gexplo.2016.06.007
Ni, N., Song, Y., Shi, R. Y., Liu, Z. T., Bian, Y. G., Wang, F., Yang, X. L., Gu, C. G., & Jiang, X. (2017). Biochar reduces the bioaccumulation of PAHs from soil to carrot (Daucus carota L.) in the rhizosphere: a mechanism study. Science of the Total Environment, 601, 1015–1023. https://doi.org/10.1016/j.scitotenv.2017.05.256
Oen, A. M. P., Beckingham, B., Ghosh, U., Kruså, M. E., Luthy, R. G., Hartnik, T., Henriksen, T., & Cornelissen, G. (2012). Sorption of organic compounds to fresh and fieldaged activated carbons in soils and sediments. Environmental Science and Technology, 46(2), 810–817. https://doi.org/10.1021/es202814e
Oleszczuk, P., Hale, S. E., Lehmann, J., & Cornelissen, G. (2012). Activated carbon and biochar amendments decrease pore-water concentrations of polycyclic aromatic hydrocarbons (PAHs) in sewage sludge. Bioresource Technology, 111, 84–91. https://doi.org/10.1016/j.biortech.2012.02.030
Pan, B., Ning, P., & Xing, B. (2008). Part IV-sorption of hydrophobic organic contaminants. Environmental Science and Pollution Research International, 15, 554–564. https://doi.org/10.1007/s11356-008-0051-y
Qin, G., Gong, D., & Fan, M. Y. (2013). Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. International Biodeterioration and Biodegradation, 85, 150–155. https://doi.org/10.1016/j.ibiod.2013.07.004
Rad, R. M., Omidi, L., Kakooei, H., Golbabaei, F., Hassani, H., Loo, R. A., & Azam, K. (2014). Adsorption of polycyclic aromatic hydrocarbons on activated carbons: kinetic and isotherm curve modeling. Int. J. Occup. Hyg., 6(1), 43–49
Rhodes, A. H., McAllister, L. E., & Chen, R. R. (2010). Impact of activated charcoal on the mineralisation of C-14-phenanthrene in soils. Chemosphere, 79(4), 463–469. https://doi.org/10.1016/j.chemosphere.2010.01.032
Salloum, M. J., Chefetz, B., & Hatcher, P. G. (2002). Phenanthrene sorption by aliphatic-rich natural organic matter. Environmental Science and Technology, 36, 1953–1958. https://doi.org/10.1021/es015796w
Scherdel, C., Reichenauer, G., & Wiener, M. (2010). Relationship between pore volumes and surface areas derived from the evaluation of N2-sorption data by DR-. BET-and t-plot. Microporous Mesoporous Mater., 132(3), 572–575. https://doi.org/10.1016/j.micromeso.2010.03.034
Sherafatmand, M., & Ng, H. Y. (2015). Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresource Technology, 195, 122–130. https://doi.org/10.1016/j.biortech.2015.06.002
Srihari, V., & Das, A. (2008). Comparative studies on adsorptive removal of phenol by three agro-based carbons: equilibrium and isotherm studies. Ecotoxicology and Environmental Safety, 71(1), 274–283. https://doi.org/10.1016/j.ecoenv.2007.08.008
Sun, D., Li, X., & Lou, L. (2010). On the research of adsorption of polycyclic aromatic hydrocarbons (Phenanthrene) in soil-groundwater in Zhangshi Irrigation District. Procedia Environmental Sciences, 2, 824–831. https://doi.org/10.1016/j.proenv.2010.10.093
Sun, Y., Yang, S., Zhao, G., Wang, Q., & Wang, X. (2013). Adsorption of polycyclic aromatic hydrocarbons on graphene oxides and reduced graphene oxides. Chemistry - An Asian Journal, 8(11), 2755–2761. https://doi.org/10.1002/asia.201300496
Sushkova, S., Minkina, T., Deryabkina, I., Mandzhieva, S., Zamulina, I., Bauer, T., Vasilyeva, G., Antonenko, E., Rajput, V., & Kızılkaya, R. (2018a). Features of accumulation, migration and transformation of benzo[a]pyrene in soil-plant system in a model condition of soil contamination. Journal of Soils and Sediments, 18(6), 2361–2367. https://doi.org/10.1007/s11368-016-1634-8
Sushkova, S., Deryabkina, I., Antonenko, E., Kizilkaya, R., Rajput, V., & Vasilyeva, G. (2018b). Benzo[a]pyrene degradation and bioaccumulation in soil-plant system under artificial contamination. Science of the Total Environment, 633, 1386–1391. https://doi.org/10.1016/j.scitotenv.2018.03.287
Sushkova, S., Minkina, T., Deryabkina, I., Rajput, V., Antonenko, E., Nazarenko, O., Yadav, B. K., Hakki, E., & Mohan, D. (2019). Environmental pollution of soil with PAHs in energy producing plants zone. Sci. Total Enviro., 655, 232–241. https://doi.org/10.1016/j.scitotenv.2018.11.080
Speciale, A., et al. (2018). Experimental exposure of blue mussels (Mytilus galloprovincialis) to high levels of benzo [a] pyrene and possible implications for human health. Ecotoxicology and Environmental Safety, 150, 96–103. https://doi.org/10.1016/j.ecoenv.2017.12.038
Usman, M., Hanna, K., & Haderlein, S. (2016). Fenton oxidation to remediate PAHs in contaminated soils: a critical review of major limitations and counter-strategies. Science of the Total Environment, 569, 179–190
U.S.E.P. Agency (2009). National Primary Drinking Water Regulations, EPA, 816-F-09–0004. http://water.epa.gov/drink/contaminants/
Valderrama, C., Gamisans, X., Cortina, J. L., Farrán, A., & De las Heras F.X. . (2009). Evaluation of polyaromatic hydrocarbon removal from aqueous solutions using activated carbon and hyper-crosslinked polymer (Macronet MN200). Journal of Chemical Technology and Biotechnology, 84, 236–245. https://doi.org/10.1002/jctb.2030
Valderrama, C., Gamisans, X., & de las Heras, X., Farrán, A., Cortina, J.L. . (2008). Sorption kinetics of polycyclic aromatic hydrocarbons removal using granular activated carbon: intraparticle diffusion coefficients. Journal of Hazardous Materials, 157, 386–396. https://doi.org/10.1016/j.jhazmat.2007.12.119
Vasilyeva, G. K., Kreslavski, V. D., Shea, P. J., & Oh, B.-T. (2001). Potential of activated carbon to decrease 2,4,6-trinitritoluene toxicity and accelerate soil decontamination. Environmental Toxicology and Chemistry, 20(5), 965–971. https://doi.org/10.1002/etc.5620200505
Vasilyeva, G. K., Strijakova, E. R., Nikolaeva, S. N., Lebedev, A. T., & Shea, P. J. (2010). Dynamics of PCB removal and detoxification in historically contaminated soils amended with activated carbon. Environmental Pollution, 158(3), 770–777. https://doi.org/10.1016/j.envpol.2009.10.010
Walters, R. W., & Luthy, R. G. (1984). Equilibrium adsorption of polycyclic aromatic hydrocarbons from water onto activated carbon. Environmental Science and Technology, 18, 395–403. https://doi.org/10.1021/es00124a002
Wang, M., Hearon, S. E., Johnson, N. M., & Phillips, T. D. (2019). Development of broad-acting clays for the tight adsorption of benzo[a]pyrene and aldicarb. Applied Clay Science, 168, 196–202. https://doi.org/10.1016/j.clay.2018.11.010
Warnock, D. D., Lehmann, J., Kuyper, T. W., & Rillig, M. C. (2007). Mycorrhizal responses to biochar in soil—concepts and mechanisms. Plant and Soil, 300, 9–20. https://doi.org/10.1007/s11104-007-9391-5
Wu, H., Wang, M., Zhu, S., Xie, J., Preis, S., Lie, F., & Wei, C. (2019). Structure and function of microbial community associated with phenol co-substrate in degradation of benzo[a]pyrene in coking wastewater. Chemosphere, 228, 128–138. https://doi.org/10.1016/j.chemosphere.2019.04.117
Xing, B. (1997). The effect of the quality of soil organic matter on sorption of naphthalene. Chemosphere, 35, 633–642. https://doi.org/10.1016/S0045-6535(97)00125-2
Yakout, S. M., Daifullah, A. A., & El-Reefy, S. A. (2013). Adsorption of naphthalene, phenanthrene and pyrene from aqueous solution using low cost activated carbon derived from agricultural wastes. Adsorption Science & Technology, 31, 293–302. https://doi.org/10.1260/0263-6174.31.4.293
Yang, K., Zhu, L. Z., & Xing, B. S. (2006). Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environmental Science and Technology, 40, 1855–1861. https://doi.org/10.1021/es052208w
Yang, X., Li, J., Wen, T., Ren, X., Huang, Y., Wang, X. (2013). Adsorption of naphthalene and its derivatives on magnetic graphene composites and the mechanism investigation. Colloids Surf., A., 422, 118–125. https://doi.org/10.1016/j.colsurfa.2012.11.063
Yang, X., Li, Z., Liu, Y., Xing, Y., Wei, J., Yang, B., Zhang, C., Yang, R. T., & Tsai, C.-J. (2019). Research progress of gaseous polycyclic aromatic hydrocarbons purification by adsorption. Aerosol Air Qual. Res., 19(4), 911–924. https://doi.org/10.4209/aaqr.2018.11.0398
Yuan, M., Tong, S., Zhao, S., & Jia, C. Q. (2010). Adsorption of polycyclic aromatic hydrocarbons from water using petroleum coke-derived porous carbon. Journal of Hazardous Materials, 181, 1115–1120. https://doi.org/10.1016/j.jhazmat.2010.05.130
Zhang, Y., Tao, S., Shen, H., Ma, J. (2009a). Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proceedings of the National Academy of Sciences, 106, 21063–21067. https://doi.org/10.1073/pnas.0905756106.
Zhang, J., He, M., Shi, Y. (2009b). Comparative sorption of benzo[a]pyrene to different humic acids and humin in sediments. Journal of Hazardous Materials,, 166, 802–809.https://doi.org/10.1016/j.jhazmat.2008.11.121
Zhang, M., Ahmad, M., Lee, S. S., Xu, L. H., & Ok, Y. S. (2014). Sorption of polycyclic aromatic hydrocarbons (PAHs) to lignin: effects of hydrophobicity and temperature. Bulletin of Environment Contamination and Toxicology, 93(1), 84–88. https://doi.org/10.1007/s00128-014-1290-x
Zhang, X., Gao, B., Creamer, A. E., Cao, C., & Li, Y. (2017). Adsorption of VOCs onto engineered carbon materials: a review. Journal of Hazardous Materials, 338, 102–123. https://doi.org/10.1016/j.jhazmat.2017.05.013
Zhao, G. X., Li, J. X., & Wang, X. K. (2011). Kinetic and thermodynamic study of 1-naphthol adsorption from aqueous solution to sulfonated graphene nanosheets. Chemical Engineering Journal, 173, 185–190. https://doi.org/10.1016/j.cej.2011.07.072
Acknowledgments
The research was financially supported by the Russian Science Foundation (project no. 20-14-00317).
Author information
Authors and Affiliations
Contributions
Tatiana Minkina: Conceptualization, Formulation of a Research Problem, Writing. Galina Vasilyeva: Data Curation, Writing—Reviewing. Yana Popileshko: Conducting Experiments. Tatiana Bauer: Writing. Svetlana Sushkova: Preparation of Biochar. Aleksey Fedorenko: Visualization. Elena Antonenko: Conducting Experiments. David Pinskii: Methodology. Mahmoud Mazarji: Writing—Review & Editing. Carla Sofia Santos Ferreira: Writing—Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest in this work.
Ethics approval
It is not applicable since the manuscript has not been involved in the use of any animal or human data or tissue.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Minkina, T., Vasilyeva, G., Popileshko, Y. et al. Sorption of benzo[a]pyrene by Chernozem and carbonaceous sorbents: comparison of kinetics and interaction mechanisms. Environ Geochem Health 44, 133–148 (2022). https://doi.org/10.1007/s10653-021-00945-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-00945-8