Abstract
In view of the high incidence rate of urinary stones in the south and southwest of Iran, this paper investigates trace elements content including heavy metals in 39 urinary stones, collected from patients in Fars province, Iran. The mineralogy of the stones is investigated using X-ray diffractometry. The samples are classified into five mineral groups (calcium oxalate, uric acid, cystine, calcium phosphate and mixed stone). Major and trace elements in each group were determined using ICP-MS method. P and Ca constitute the main elements in urinary stones with Ca being more affine to oxalates while other alkali and alkaline earths precipitate with phosphate. Significant amounts of trace elements, especially Zn and Sr, were found in urinary calculi (calcium oxalate and phosphates) relative to biominerals (uric acid and cystine). Among urinary calculi, calcium phosphate contains greater amounts of trace metal than calcium oxalate. Phosphates seem to be the most important metal-bearing phases in urinary stones. Results indicate that concentrations of elements in urinary stones depend on the type of mineral phases. Significant differences in elements content across various mineralogical groups were found by applying statistical methods. Kruskal–Wallis test reveals significant difference between Ca, P, K, Na, Mg, S, Zn, Sr, Se, Cd, and Co content in different investigated mineral groups. Moreover, Mann–Whitney test differentiates Ca, Na, Zn, Sr, Co, and Ni between minerals in oxalate and uric acid stones. This study shows that urinary stone can provide complementary information on human exposure to elements and estimate the environmental risks involved in urinary stones formation.
Similar content being viewed by others
Introduction
Urinary stone formation in the urinary tract of the human body is the most common and painful urological disorder throughout the world (Singh et al. 2009; Stamatelou et al. 2003). Urinary stones, which mostly form in kidneys (Evan 2010), are products of the pathological bio-mineralization processes in the urinary system (Kuta et al. 2012; Giannossi and Summa 2013). The major constituents of the stones include bio-minerals and different inorganic substances, with crystalline or amorphous structures (Hesse et al. 2009).
Urinary stone disease is a multi-factorial health problem with no simple solution from the medical prevention of stone recurrence (Robertson et al. 2010). Kidney stones generally form in response to environmental and/or metabolic risk factors (Brikowski et al. 2008). The following factors may play a role in kidney stone formation: environment, nutrition, gender, geography, fluid intake, climate, associated diseases, and socio-economic status (Golovanova et al. 2006; Safarinejad 2007; Pourmand and Pourmand 2012; Pearle and Lotan 2012). The prevalence of renal stone disease has been rising in both sexes, and is 2–3 times more common in males than in females (Dajani et al. 1988; Andrew and Chandru 2001; Stamatelou et al. 2003). Peak incidence rate occurs in the second or third decade of life (Shokouhi et al. 2008). The lifetime prevalence of kidney stone disease is estimated at 1–15 % (Pearle and Lotan 2012).
Genetic factors and dietary influences is said to outweigh climatic effects (Pearle and Lotan 2012). Dietary factors include a high intake of animal proteins and oxalates and a low intake of potassium-bearing citrus fruits and fluids (Tur et al. 1991). A person with a family history of kidney stones is more likely to develop urinary stones (Abboud 2008c).
Mineralogical studies of urinary stones and the involved crystallization processes are particularly important in understanding possible environmental and metabolic factors that lead to stone formation. Early studies on urinary stones have revealed some information on composition, mineralogy, structure, processes of formation and geo-environmental factors (Ackermann et al. 1988; Bellizzi et al. 1999; Kohri et al. 1989). Urinary stones consist of about 40 components (Bazin et al. 2012; Hesse 1988). Major chemical constituents of calculi stones are calcium phosphate (hydroxyapatite), calcium oxalates (whewellite and weddellite) and Urates (Nasir et al. 2004; Evan 2010). Struvite stone is a less common type caused by the urinary tract infection. Cystine is also a rare stone (Giannossi et al. 2013). Uric acid stone is a less common stone (Abboud 2008c). Physiologically, urinary calculus formation is generally caused by disturbed urinary compositions with altered urinary pH, and increased concentrations of lithogenic components as, e.g., calcium, oxalate, phosphate, and lack of inhibitoric substances such as citrate and magnesium (Beging et al. 2010). Calcium oxalate is the most frequent mineral phase found in uroliths with a frequency of approximately 70–75 % (Schubert 2006).
In recent years, the significance and role of trace elements in biological lithogenesis have received increasing attention. Although trace elements are necessary for the optimal development and metabolic functioning of all living organisms, many researchers believe that they are also essential mediators in the development and progression of various diseases (Mertz 1981; Fraga 2005; Failla 2003; Houtman 1996; Navarro and Rohan 2007). Trace elements also play a role in crystal formation and/or stone organization owing to their special affinity for some crystalline phases (Slojewski et al. 2010; Touryan et al. 2004; Bazin et al. 2008). Some trace elements may influence the external morphology of growing crystals and accelerate or slow down the crystallization process (Slojewski 2011; Fang et al. 2005; Munoz and Valiente 2005). However, metal content in urinary stones would reflect exposure of an organism to these metals analogous to other biological matrices used for human biomonitoring of metals (e.g., blood, hair, or urine; Esteban and Castaño 2009).
Of the several aforementioned factors, environmental factors are suspected to have a direct relationship to the composition of urine, which is governed mainly by diet and drinking water composition (Chandrajith et al. 2006). Also, the geographic distribution of stone disease tends to roughly follow environmental risk factors; a higher prevalence of stone disease is usually found in hot, arid, or dry climates such as the mountains, desert, or tropical areas (Pearle and Lotan 2012; Zarasvandi et al. 2013). In certain areas of the world, such as the Afro-Asian stone belt, including the Middle East, and countries located in tropical and subtropical areas, a high incidence rate of urolithiasis is reported (Abboud 2008c).
The prevalence of renal stones in Iran is reported to be 5.7 % (Safarinejad 2007), and varies in different parts of the country (Zarasvandi et al. 2013). The regional distribution indicates a higher incidence rate in the southcentral (Kerman and Hormozgan) and southwest (Fars, Boushehr, Khouzestan, Ilam, and Chaharmahal provinces) of the country (Pourmand and Pourmand 2012), with an increasing trend from west to east and from north to south (Safarinejad 2007).
Fars province, southwest of Iran (50–55°E and 27–31°N) have different climatic regions. These include north and northwest mountainous area with moderately cold winters and mild summers; the central regions, with relatively rainy mild winters and hot dry summers and south and southeast region with mild winters and very hot summers. Maximum annual temperatures ranges between 35 and 40 °C (in August), while minimum annual temperatures ranges from 2 to −7 °C (in December). Annual rainfall varies from 100 mm in the south to 200–400 mm in the north.
Careful study of elements association with minerals or mineral groups is therefore crucial in assessing the effect of other factors on trace metal content (Kuta et al. 2013). Urinary stone analysis is also important in determining the possible etiology and pathophysiology of stone formation, which in turn may help prevent urinary stone formation (Jing et al. 2010).
This paper describes the results of chemical analysis of 39 urinary stones samples. The main purpose is to determine associated elements with minerals in order to establish the most important factors affecting minor elements content in urinary stone.
Materials and methods
Eighty-three samples were collected between April 2013 and August 2013 from patients undergoing surgery for urinary stone removal (57 males and 26 females). By a single surgeon (D.I.) at Shahid Faghihi Hospital, Shiraz City. Each patient was asked to fill out a questioner prior to his/her surgery regarding personal details including gender, age, medical history (such as weight, personal and family anamnesis), address, and other relevant social information.
The removed stones were placed in polyethylene dry bottles and kept cool until analysis. All polyethylene bottles were labeled with the name, sex, and age of the patient and the date of surgery.
Thirty-nine urinary stone samples with different morphologies were washed several times with deionized water to free the stones of urine, blood stains, and remnants of organic matter, and finally were rinsed with distilled water. After drying, each stone was ground in an agate mortar and homogenized.
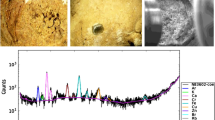
A fraction was sent to Kansaran Binaloud Lab, Tehran, for X-ray diffraction analysis (XRD) to obtain the crystalline phase composition of the urinary stones. The instrument used a nickel-filtered Cu-Kα with a generator voltage of 40 kV and generator current of 30 mA. Qualitative interpretation of the diffractometric profiles was achieved by comparing the peaks of reflection with PDF card (Powder Diffraction File). Oxalate, Phosphate, uric acid, and cystine minerals were detected in 36 renal, two bladders, and one ureteral stone samples.
Elements concentration was determined using an inductively coupled plasma-mass spectrometry (ICP-MS). A 0.25-g sample was heated in HNO3–HClO4–HF at 200 °C in hot box for 4–5 h. The residue was dissolved in HCl. Following digestion, urinary stones were completely dissolved. As the digestion was carried out in encapsulated vessels, it is assumed that there was no loss of volatile elements. In this method, QA/QC included reagent blanks and analytical duplicates, and analysis of the standard reference material (multi-element CRM such as OREAS 70b, OREAS 73b, GBW 07302, GBW 07305) are used. Data was processed statistically using SPSS statistical package version 19.0 for Windows. Mann–Whitney U test and Kruskal–Wallis ANOVA were used for descriptive statistics. The correlation between elements is described by Spearman correlation coefficient.
Results and discussion
The collected urinary stones are varied in terms of patients’ age and may be divided into four groups: <40 years old (21 samples), 40–50 years old (20 samples), 50–60 years old (29 samples) and >60 years old (13 samples). Urinary stones were found to be more frequent in men, with increased prevalence in the 50–60 years old group. Also, the information taken from the questionnaires showed hypertension (9 samples), diabetes (4 samples) and family history of urinary stone (14 samples) the study group. Patients with a family history of the disease were infrequent. The most frequent co-existing diseases were hypertension and diabetes, as already shown by Basiri et al. (2010).
Table 1 represents the mineralogical composition of 39 samples according to mineralogical composition. The samples may be classified into five mineral groups (Table 2). The most common group (44 %) comprises mixed stones involving a mixture of calcium oxalate and uric acid. The second largest group is calcium oxalate stones (26 %), while (20 %) belong to uric acid (uricite) group. Each of the phosphate (as hydroxyapatite) and cystine group of stones also comprises 5 % of the urinary stones. Figures 1, 2, 3, and 4 show X-ray diffraction pattern of some urinary stone samples.
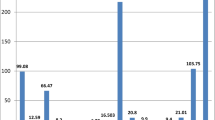
Table 3 displays the results of the chemical analysis and mean concentrations of each calculi type. Calcium has the highest concentration, ranging between 0.04 and 43.46 % and averaging 14.76 %. Calcium is the main constituent in all types of stones. The high Ca content in urinary stones probably reflects a Ca-rich diet such as diary and milk products, red meat, tea, egg, and hard water as already noted by Robertson et al. (1980) and Sobhi (2006), or pathologic conditions such as renal leak hypercalciuria, absorptive or resorptive and idiopathic hypercalciuria.
Phosphorous is the major constituent in phosphate stones. The P content in all stone samples ranges from 0.01 to 14.45 % and averages 0.95 % (Table 3). Also, the reason for high P content in some urinary stones can be the excessiveness of drinking milk and eating cheese, egg, and some vegetables with high phosphorous content is high concentration of P in water (Abboud 2008a), and pathologic conditions like renal tubular acidosis (RTA) type 1, hyperparathyroidism, medullary strong kidneys, and any condition causing metabolic acidosis and alkaline urine. The highest and lowest P content occurs in phosphate and cystine calculi minerals, respectively.
Na content ranges between 0.01 and 0.86 % while K comprises 0.11–0.22 %, respectively. Average Na and K content is 0.15 and 0.13 %, respectively (Table 3). Na and K normally occur at high concentrations in urine (approximately 150 mmol l−1 for Na and 50 mmol l−1 for K in urine; Kuta et al. 2013).
Calculated correlation coefficients are presented in Table 4. Na (r = 0.572) is positively correlated with phosphorous, indicating the strong affinity of alkali metals to phosphate in urinary stones (Chandrajith et al. 2006). Close association of Na to hydroxylapatite is quite natural. A high correlation coefficient between Na and Ca (r = 0.633) reflects that Na replaces Ca in many rock-forming minerals, for example, plagioclases (albite-anorthite series NaAl3Si3O8-CaAl2Si2O8), because of their very similar ionic radius (Shannon and Prewitt 1969). The low correlation between K and Ca (r = 0.194) in urinary stones reflects the fact that K and Ca have different geochemical properties.
Mg content in the analyzed stones ranges from 0.004 to 0.79 % averaging 0.06 % (Table 3). A positive correlation for Mg and P (r = 0.767) indicates the tendency of Mg to enter phosphate minerals structure. Mg is an important element in biological calcification (Atakan et al. 2007) and its presence in urinary stones is usually an indicator of an increase in concentration in the human body (Deeming and Weber 1977). The exact role of Mg in kidney stone formation is yet to be fully explained (Singh et al. 2009; Kohri et al. 1988). Apparently, food and hard water are responsible for Mg content along with some medicines and drugs (Giannossi et al. 2013), and Mg is a major component of struvite (infected) stones. In this stone, mg is precipitated with ammonium and phosphate in alkaline urine.
S content (0.03–35.90 %) is characteristic of the cystine group of stones. Concentrated Fe in urine usually comes from cells in the human urine channel (Lentner 1981), and increases in percentage in the urinary system and allows to be incorporated in oxalate and calcium phosphate stones (Wandt and Underhill 1988). Fe ranges between 37 and 204 ppm, averaging 82.33 ppm. Fe content is the result of the inhibitory properties of Fe3+ on calcium oxalate crystallization as suggested by Meyer and Thomas (1982) and (Munoz and Valiente 2005).
Trace elements such as Zn and Sr are present in high concentrations, averaging 83 and 141 ppm, respectively (Table 3). The highest Zn and Sr concentrations occur in phosphatic calculi minerals. Table 4 shows the relationship between Sr and Zn (r = 0.823).
Wandt and Underhill (1988) already indicated that Zn positively correlations with Sr, and predominantly associates with hydroxylapatite, a fact substantiated by positive correlations between Zn and P content of the stones (Abboud 2008a; Zarasvandi et al. 2013).
In the current study, Zn accumulations in hydroxylapatite support the relationship between Zn and P (r = 0.761) in the urinary stones. However, a positive correlation of Sr and P (r = 0.748) reflects the close natural relation between Sr and P (Abed and Abdalla 1998). Zn and Sr content in mixed stones also reflects the presence of the respective phases in urinary stones. For example, mixed calcium oxalate/uric acid stones contain moderate amounts of heavy metals (Bazin et al. 2007). Al content (82–504 ppm) is also relatively high when compared with other elements.
Sutor (1969) found that a small amount of Al3+ can remove oxalate ions and thus prevent crystallization of calcium oxalate. Low concentrations of toxic trace elements, including Pb and V, range between 2 and 39 ppm, and 6 and 10 ppm, respectively. Average Pb and V content is 12.1 and 8.3 ppm, respectively. Pb is a consequence of high levels of Pb in the body. The presence of this element strongly indicates environmental pollution (Giannossi et al. 2013). Bazin et al. (2007) reported decreased levels of Pb in urinary stones, correlating with decreased available Pb in the environment. The role of Pb in biolithogenesis is not yet fully known, but some authors have found a correlation between lead in stones and urine, which may lead to the conclusion that Pb may also play some role in the crystallization of calculi stones in the urinary tract (Słojewski et al. 2010).
Selenium occurs only in trace amounts (<12.15 ppm) in analyzed stones, with the exception of Uricite stones in which selenium ranges from 0.05 to 40.01 ppm and averages 5.09 ppm. The role of Se in biolithogenesis is poorly documented. However, it is suggested that like other semimetal, selenium may have some interactions with stone constituents, or be captured in the structure of crystals incidentally (Bazin et al. 2007; Sakly et al. 2003). The highest Se content (40.01 ppm) was found in the patient who suffers from diabetes. It has been suggested that higher levels of selenium taken from supplements or received naturally are associated with an increased risk of diabetes (Stranges et al. 2007). Cadmium is also suggested as a risk factor in urinary tract stone formation (Perk et al. 2002). Only a few studies in the medical literature deal with the role of Cd in biolithogenesis. It is suggested that cadmium might have some inhibitory effect on calcium oxalate crystallization (Hofbauer et al. 1991). Furthermore, some heavy metals including Mo, Cd, Co, Rb, Ni, Cu, Mn, and As also display considerable concentration (Table 3).
Values below detection limits were assumed to be 1/2 of detection limits to compare the differences in elements content in Kruskal–Wallis and Mann–Whitney tests and also calculate correlation coefficients.
Statistical comparison of elemental content, and minerals groups was carried out using Kruskal–Wallis ANOVA. P values lower than 0.05 are considered to indicate significant differences. For measured elements (Ca, P, K, Na, Mg, S, Zn, Sr, Ni, Se, Ba, Cd, Co), obvious differences with particular mineral components, and a confidence level of 95 %, was observed. Other elements did not show significant differences (Table 5). However, some differences between mineral groups are obvious.
Mann–Whitney U, p < 0.05 test was used to confirm the results of Kruskal–Wallis test. This test shows the differences between the elements and different mineral groups. For instance, Na, Zn and Sr (p = 0.00), Ca (p = 0.001), Co (p = 0.003), and Ni (p = 0.009) show significant differences between mineral groups, with high concentrations in oxalate stones. Se (p = 0.009) also shows a significant difference between the two mineral groups of oxalate and uric acid, whereas, no significant differences were found between K (p = 0.46), Cd and Ba (p = 0.573). Most Cd differences (p = 0.01 and p = 0.027) occur between the cystine and oxalate groups (Table 6). These differences may be due to the presence of Ca in the structure of calcium oxalate minerals such as whewellite and weddellite. High Cd concentration (0.93 ppm) also occurs in oxalate stones.
The results of this study confirm the conclusions previously described by other authors. Among calcium stones, calcium phosphate calculi contained a greater amount of metals than calcium-oxalate calculi (Giannossi et al. 2013). This is especially true for Sr and Zn, when compared with calcium oxalate stones. Fe, Rb, and Pb are also more abundant in calcium stones than in uric acid or cystine stones (Słojewski et al. 2010). Bazin et al. (2007), showed high Zn and Sr content in phosphatic stones, and contrary to Joost and Tessadri (1986) a lower proportion in calcium oxalate stones. Levinson (Levinson et al. 1978) observed a considerably higher Zn and Sr content in calcium oxalate and calcium phosphate stones than in uric acid and cystine urinary stones. Strong correlations between Zn, Sr, Ni, and P content are also observed in the work of Abboud (2008a).
Table 7 compares the mean concentration of elements in the present study with previous ones carried out in different geological areas (Kuta et al. 2013; Abboud 2008b; Zarasvandi et al. 2013; Chandrajith et al. 2006; Giannossi et al. 2013). The difference between elements distribution in the clusters of urinary stones collected from different regions is most likely associated with geology, drinking water quality, diet, and therapeutic facilities (Golovanova et al. 2006; Abboud 2008b). Therefore, it is not surprising that collected urinary stones from Fars province have their own differences with other parts of the world such as Jordan, Sri Lanka, and Italy.
Conclusions
Essential trace elements for specific metabolic processes are temporally stored and excreted via the kidneys. In this process, the elements may result in the formation of urinary stones in the urinary tract. Ultimate analysis of urinary stones has previously been used to identify metal content in urinary stones. However, the results of this study show that the content of elements is significantly affected by mineralogical composition of the calculi. Calcium stones contain the highest proportion of metals, especially Sr and Zn. Trace amounts of metals are also found in uric acid or cystine stones. The reason for the observed difference in the metal content in different types of stones is apparently the similarity between the charge and size of Zn and Sr ions with calcium ion, which allows these elements to substitute for calcium in the crystal lattices. Se content in uric acid stones is also significant. Also, Zn and Sr accumulation in phosphatic stones supports the observed correlation between these elements and P in the urinary stones. However, geochemical similarity between various elements is only one effective factor in the formation of urinary stones. The results of statistical tests revealed significant elemental differences between the five assigned mineralogical groups of urinary stones. A high concentration of one or more elements in urinary stones with different mineralogical is probably due to various reasons such as different chemical behavior of elements, along with metabolic and environmental factors. Thus, the evaluation of elements content in different types of urinary stone should always be considered along with their mineralogical composition. Such data could help the urologist to understand the causes of the formation of urinary stones and ultimately to plan safer methods to avoid their formation and suggest dietary regimes to stop their inception.
References
Abboud, I. A. (2008a). Analyzing correlation coefficients of the concentrations of trace elements in urinary stones. Jordan Journal of Earth and Environmental Sciences, 1(2), 73–80.
Abboud, I. A. (2008b). Concentration effect of trace metals in Jordanian patients of urinary calculi. Environmental Geochemistry and Health, 30(1), 11–20.
Abboud, I. A. (2008c). Mineralogy and chemistry of urinary stones: patients from North Jordan. Environmental Geochemistry and Health, 30(5), 445–463.
Abed, A. M., & Abdalla, R. S. (1998). On the state of weathering of the Upper Cretaceous red phosphorites of Eshidiya, southern Jordan. Journal of African Earth Sciences, 27(1), 39–54.
Ackermann, D., Baumann, J. M., Futterlieb, A., & Zingg, E. J. (1988). Influence of calcium content in mineral water on chemistry and crystallization conditions in urine of calcium stone formers. European Urology, 14(4), 305–308.
Andrew, J. P. & Chandru, P. S. (2001). Diagnosis and initial management of kidney stones. American Family Physician, http://www.aatp.org/atp/20010401/1329.html. p. 17.
Atakan, I. H., Kaplan, M., Seren, G., Aktoz, T., Gül, H., & Inci, O. (2007). Serum, urinary and stone zinc, iron, magnesium and copper levels in idiopathic calcium oxalate stone patients. International Urology and Nephrology, 39(2), 351–356.
Basiri, A., Shakhssalim, N., Khoshdel, A. R., Ghahestani, S. M., & Basiri, H. (2010). The demographic profile of urolithiasis in Iran: a nationwide epidemiologic study. International Urology and Nephrology, 42(1), 119–126.
Bazin, D., Carpentier, X., Traxer, O., Thiaudiere, D., Somogyi, A., Reguer, S., et al. (2008). Very first tests on SOLEIL regarding the Zn environment in pathological calcifications made of apatite determined by X-ray absorption spectroscopy. Journal of Synchrotron Radiation, 15(5), 506–509.
Bazin, D., Chevallier, P., Matzen, G., Jungers, P., & Daudon, M. (2007). Heavy elements in urinary stones. Urological Research, 35(4), 179–184.
Bazin, D., Daudon, M., Combes, C., & Rey, C. (2012). Characterization and some physicochemical aspects of pathological microcalcifications. Chemical Reviews, 112(10), 5092–5120.
Beging, S., Mlynek, D., Hataihimakul, S., Poghossian, A., Baldsiefen, G., Busch, H., et al. (2010). Field-effect calcium sensor for the determination of the risk of urinary stone formation. Sensors and Actuators B: Chemical, 144(2), 374–379.
Bellizzi, V., DeNicola, L., Minutolo, R., Russo, D., Cianciaruso, B., Andreucci, M., et al. (1999). Effects of water hardness on urinary risk factors for kidney stones in patients with idiopathic nephrolithiasis. Nephron, 81(Suppl. 1), 66–70.
Brikowski, T. H., Lotan, Y., & Pearle, M. S. (2008). Climate-related increase in the prevalence of urolithiasis in the United States. Proceedings of the National Academy of Sciences, 105(28), 9841–9846.
Chandrajith, R., Wijewardana, G., Dissanayake, C., & Abeygunasekara, A. (2006). Biomineralogy of human urinary calculi (kidney stones) from some geographic regions of Sri Lanka. Environmental Geochemistry and Health, 28(4), 393–399.
Dajani, A., Khadra, A. A., & Baghdadi, F. (1988). Urolithiasis in Jordanian Children.: A Report of 52 Cases. British Journal of Urology, 61(6), 482–486.
Deeming, S., & Weber, C. (1977). Evaluation of hair analysis for determination of zinc status using rats. The American Journal of Clinical Nutrition, 30(12), 2047–2052.
Esteban, M., & Castaño, A. (2009). Non-invasive matrices in human biomonitoring: a review. Environment International, 35(2), 438–449.
Evan, A. P. (2010). Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatric Nephrology, 25(5), 831–841.
Failla, M. L. (2003). Trace elements and host defense: recent advances and continuing challenges. The Journal of Nutrition, 133(5), 1443S–1447S.
Fang, X., Ahmad, S. R., Mayo, M., & Iqbal, S. (2005). Elemental analysis of urinary calculi by laser induced plasma spectroscopy. Lasers in Medical Science, 20(3–4), 132–137.
Fraga, C. G. (2005). Relevance, essentiality and toxicity of trace elements in human health. Molecular Aspects of Medicine, 26(4), 235–244.
Giannossi, M. L., & Summa, V. (2013). An observation on the composition of urinary calculi: environmental influence. Medical geochemistry (pp. 67–90). Netherlands: Springer.
Giannossi, M. L., Summa, V., & Mongelli, G. (2013). Trace element investigations in urinary stones: A preliminary pilot case in Basilicata (Southern Italy). Journal of Trace Elements in Medicine and Biology, 27(2), 91–97.
Golovanova, O., Palchik, N., Maksimova, N., & In, A. (2006). Comparative characterization of the microelement composition of kidney stones from patients in the Novosibirsk and Omsk regions.
Hesse, A., & Sanders, G. (1988). Atlas of infrared spectra for the analysis of urinary concrements. Stuttgart: Georg Thieme.
Hesse, A., Tiselius, H. G., Siener, R., & Hoppe, B. (2009). Urinary stones: diagnosis, treatment, and prevention of recurrence. 3rd revised and enlarged edition. Karger: Basel.
Hofbauer, J., Steffan, I., Höbarth, K., Vujicic, G., Schwetz, H., Reich, G., et al. (1991). Trace elements and urinary stone formation: new aspects of the pathological mechanism of urinary stone formation. The Journal of Urology, 145(1), 93–96.
Houtman, J. P. (1996). Trace elements and cardiovascular diseases. Journal of Cardiovascular Risk, 3(1), 18–24.
Jing, Z., GuoZeng, W., Ning, J., JiaWei, Y., Yan, G., & Fang, Y. (2010). Analysis of urinary calculi composition by infrared spectroscopy: a prospective study of 625 patients in eastern China. Urological Research, 38(2), 111–115.
Joost, J., & Tessadri, R. (1986). Trace element investigations in kidney stone patients. European Urology, 13(4), 264–270.
Kohri, K., Garside, J., & Blacklock, N. (1988). The role of magnesium in calcium oxalate urolithiasis. British Journal of Urology, 61(2), 107–115.
Kohri, K., Kodama, M., Ishikawa, Y., Katayama, Y., Takada, M., Katoh, Y., et al. (1989). Magnesium-to-calcium ratio in tap water, and its relationship to geological features and the incidence of calcium-containing urinary stones. The Journal of Urology, 142(5), 1272–1275.
Kuta, J., Machát, J., Benová, D., Červenka, R., & Kořistková, T. (2012). Urinary calculi—atypical source of information on mercury in human biomonitoring. Central European Journal of Chemistry, 10(5), 1475–1483.
Kuta, J., Machát, J., Benová, D., Červenka, R., Zeman, J., & Martinec, P. (2013). Association of minor and trace elements with mineralogical constituents of urinary stones: A hard nut to crack in existing studies of urolithiasis. Environmental Geochemistry and Health, 35, 1–12.
Lentner, C. (1981). Geigy scientific tables. English edition. Vol. 1 (Vol. 53–107). Ciba Geigy: Basel.
Levinson, A., Nosal, M., Davidman, M., Prien, E, Sr, Prien, E, Jr, & Stevenson, R. (1978). Trace elements in kidney stones from three areas in the United States. Investigative Urology, 15(4), 270.
Mertz, W. (1981). The essential trace elements. Science, 213(4514), 1332–1338.
Meyer, J. L., & Thomas, W. C, Jr. (1982). Trace metal-citric acid complexes as inhibitors of calcification and crystal growth. I. Effects of Fe(III), Cr(III) and Al(III) complexes on calcium phosphate crystal growth. The Journal of Urology, 128(6), 1372–1375.
Munoz, J. A., & Valiente, M. (2005). Effects of trace metals on the inhibition of calcium oxalate crystallization. Urological Research, 33(4), 267–272.
Nasir, S., Kassem, M. E., El-Sherif, A., Fattah, T. (2004). Physical investigation of urinary calculi: Example from the Arabian Gulf (State of Qatar). http://www.oxfordresearchforum.i12.com/articles/article% 205.htm (assessed on 12.08.2005).
Navarro Silvera, S. A., & Rohan, T. E., (2007). Trace elements and cancer risk: a review of the epidemiologic evidence. Cancer Causes Control, 18, 7–27.
Perk, H., Serel, T. A., Koşar, A., Deniz, N., & Sayin, A. (2002). Analysis of the trace element contents of inner nucleus and outer crust parts of urinary calculi. Urologia Internationalis, 68(4), 286–290.
Pourmand, G., & Pourmand, B. (2012). Epidemiology of stone disease in Iran. In urolithiasis (Vol. 85–87). London: Springer.
Robertson, W. G. (2010). Urinary tract stones in: medical therapy in urology. Springer: London.
Robertson, W. G., Peacock, M., Heyburn, P., & Hanes, F. (1980). Epidemiological risk factors in calcium stone disease. Scandinavian Journal of Urology and Nephrology, 53, 15–28.
Pearle. M. S, Yair Lotan, (2012), Urinary Lithiasis: Etiology, epidemiology, and pathogenesis in: Campbell-Walsh urology, 10thed.
Safarinejad, M. R. (2007). Adult urolithiasis in a population-based study in Iran: prevalence, incidence, and associated risk factors. Urological Research, 35(2), 73–82.
Sakly, R., Chaouch, A., El Hani, A., & Najjar, M.-F. (2003). Effects of intraperitoneally administered vitamin E and selenium on calcium oxalate renal stone formation: experimental study in rat. Paper presented at the Annales d’urologie.
Schubert, G. (2006). Stone analysis. Urological Research, 34(2), 146–150.
Shannon, R. T., & Prewitt, C. T. (1969). Effective ionic radii in oxides and fluorides. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 25(5), 925–946.
Shokouhi, B., Gasemi, K., & Norizadeh, E. (2008). Chemical composition and epidemiological risk factors of urolithiasis in Ardabil Iran. Research Journal of Biological Sciences, 3(6), 620–626.
Singh, V. K., Rai, A., Rai, P., & Jindal, P. (2009). Cross-sectional study of kidney stones by laser-induced breakdown spectroscopy. Lasers in Medical Science, 24(5), 749–759.
Słojewski, M. (2011). Major and trace elements in lithogenesis. Central European Journal of Urology, 64(2), 58–61.
Słojewski, M., Czerny, B., Safranow, K., Jakubowska, K., Olszewska, M., Pawlik, A., et al. (2010). Microelements in stones, urine, and hair of stone formers: a new key to the puzzle of lithogenesis? Biological Trace Element Research, 137(3), 301–316.
Sobhi, N. (2006). “The mineralogy and chemistry of urinary stones from the Arabian Gulf.” Internet site, work not published., Result work under proposing: 8.
Stamatelou, K. K., Francis, M. E., Jones, C. A., Nyberg, L. M., & Curhan, G. C. (2003). Time trends in reported prevalence of kidney stones in the United States: 1976–19941. Kidney International, 63(5), 1817–1823.
Stranges, S., Marshall, J. R., Natarajan, R., Donahue, R. P., Trevisan, M., Combs, G. F., et al. (2007). Effects of long-term selenium supplementation on the incidence of type 2 diabetes a randomized trial. Annals of Internal Medicine, 147(4), 217–223.
Sutor, D. (1969). Growth studies of calcium oxalate in the presence of various ions and compounds. British Journal of Urology, 41(2), 171–178.
Touryan, L. A., Lochhead, M. J., Marquardt, B. J., & Vogel, V. (2004). Sequential switch of biomineral crystal morphology using trivalent ions. Nature Materials, 3(4), 239–243.
Tur, J., Prieto, R., & Grases, F. (1991). An animal model to study the effects of diet on risk factors of calcium stone formation. Scandinavian Journal of Urology and Nephrology, 25(4), 311–314.
Wandt, M. A. E., & Underhill, L. G. (1988). Covariance biplot analysis of trace element concentrations in urinary stones. British Journal of Urology, 61(6), 474–481.
Zarasvandi, A., Heidari, M., Sadeghi, M., & Mousapoor, E. (2013). Major and trace element composition of urinary stones, Khuzestan province, Southwest, Iran. Journal of Geochemical Exploration, 131, 52–58.
Acknowledgments
This research was supported by “Shiraz University Medical Geology Research” to whom the authors are indebted. The authors would also like to express their gratitude to Zarazma Mineral Studies Company for their sample analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keshavarzi, B., Yavarashayeri, N., Irani, D. et al. Trace elements in urinary stones: a preliminary investigation in Fars province, Iran. Environ Geochem Health 37, 377–389 (2015). https://doi.org/10.1007/s10653-014-9654-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-014-9654-z