Abstract
Gold has been mined at San Antonio-El Triunfo, (Baja California Sur, Mexico) since the 18th century. This area has approximately 5,700 inhabitants living in the San Juan de Los Planes and El Carrizal hydrographic basins, close to more than 100 abandoned mining sites containing tailings contaminated with potentially toxic elements such as arsenic. To evaluate the arsenic exposure of humans living in the surrounding areas, urinary arsenic species, such as inorganic arsenic (iAs) and the metabolites mono-methylated (MMA) and di-methylated arsenic acids (DMA), were evaluated in 275 residents (18–84 years of age). Arsenic species in urine were analyzed by hydride generation-cryotrapping-atomic absorption spectrometry, which excludes the non-toxic forms of arsenic such as those found in seafood. Urinary samples contained a total arsenic concentration (sum of arsenical species) which ranged from 1.3 to 398.7 ng mL−1, indicating 33 % of the inhabitants exceeded the biological exposition index (BEI = 35 ng mL−1), the permissible limit for occupational exposure. The mean relative urinary arsenic species were 9, 11 and 80 % for iAs, MMA and DMA, respectively, in the Los Planes basin, and 17, 10 and 73 %, respectively, in the El Carrizal basin. These data indicated that environmental intervention is required to address potential health issues in this area.
Similar content being viewed by others
Introduction
Arsenic (As) exposure is a risk factor for human health, and the primary sources of human exposure are ingestion of inorganic As (iAs) contaminated water (Mazumder 2008), industrial waste which can affect food (Soleo et al. 2008), pesticides (Watson et al. 2003) and the inadequate mine waste disposal (Cho et al. 2013). Gold extraction releases huge amounts of As from ore tailings (>8,000 mg As Kg−1) and ash (50 % total As), which are distributed in the environment by fluvial and eolian processes and can cause severe health effects (Bodénan et al. 2004; Krysiak and Karczewska 2007; Lim et al. 2008).
Chronic iAs exposure can result in adverse health effects for the several million people currently exposed. Most of the toxic effects have been evaluated in people exposed to high iAs in drinking water and include skin lesions (hyperpigmentation and hyperkeratosis), which are often the first visible sign and can occur after several years of exposure (Valenzuela et al. 2005). There is also strong evidence that chronic iAs ingestion is related to cardiovascular diseases (Chen et al. 1996; Mazumder 2008), diabetes (Coronado-González et al. 2007; Del Razo et al. 2011) and cancer (Smith et al. 1992; ATSDR 2007).
Humans are exposed to iAs through water and inhalation, though iAs is absorbed more slowly after inhalation than ingestion (NRC 1999). After ingestion, the half-life of As in blood is short (1–2 h; Herbel et al. 2002) and the As is rapidly excreted (primarily in urine). The enzymatic biotransformation of iAs occurs by means of reduction and oxidative methylation reactions, yielding methyl arsenic acid (MMA) and dimethylarsenic acid (DMA) which are excreted in the urine (Hughes et al. 2011) and account for 80 % of the As eliminated from the human body (Crecelius 1977). Urine levels are considered a representative biomarker of dose and can be used to monitor environmental and occupational exposure to iAs (ATSDR 2007; Heitland and Köster 2009).
Knowledge about the type and concentration of As species in urine provides information on exposure as well as the methylation capacity for the individual(s), which is relevant because the toxic and carcinogenic actions of As are fundamentally associated with its metabolism and excretion. There is a body of evidence linking methylation capacity, and specifically high %MMA, to iAs-related disease risks. Epidemiological studies have reported an association between individual methylation patterns, specifically the proportion of MMA in urine (%MMA), and risk for several different As-related diseases including bladder cancer, skin cancer and As-caused skin lesions (Del Razo et al. 1997; Chun-Yu et al. 2000; Steinmaus et al. 2006.
In order to assess the population risk in areas surrounding gold mines, the aim of this study was to determine the urinary As levels and excretion in residents of the El Triunfo-San Antonio mining district and adjacent areas (southernmost Baja California Peninsula). This would provide background information for assessing the risk of iAs exposure to human health and to suggest actions for reducing exposure to this inorganic contaminant.
Study area
The mining district of El Triunfo-San Antonio is located at the centre of the southernmost Baja California Peninsula of Mexico, approximately 450 m above sea level (masl) and 50 km from La Paz City. Approximately 600 Tons of As2O3 is estimated to be contained in the mine waste discarded above ground, which is now exposed to weathering and erosion without any environmental remediation (Carrillo 1996). Tailings and smelting ash were transported by the streams within these two hydrographic basins (Fig. 1), the San Juan de Los Planes (SJLP) drainage basin which discharges at La Ventana bay in the Gulf of California, and the El Carrizal drainage basin which discharges into the Pacific Ocean. Four of the seven towns (San Antonio, Los Planes, Juan Domínguez Cota and El Sargento) containing ~3,156 inhabitants are located within the SJLP basin, and the other three towns (El Triunfo, El Carrizal and Melitón Albañez) containing ~2,527 inhabitants are located within the El Carrizal drainage (INEGI 2010).
The weather is desert dry, hot to warm, with summer rains, with mean annual temperature from 16 to 22 °C (Nava-Sánchez 1992). In summer, rain is due to tropical storms or sporadic hurricanes, and the precipitation (400 mm) at high altitude (>360 masl) is greater than that at lower elevations (100–200 mm) (Robles 1985). Weather influences tailing oxidation, which results in iAs contaminating streams and groundwater, where it is ultimately ingested by humans. Exposed tailings are more vulnerable to runoff and wind erosion resulting in water contamination.
Previous geochemical studies included the determination of bioavailable As in sediments from El Triunfo and San Antonio (Carrillo 1996), and potentially toxic elements (PTEs) from tailings such as As, Cd, Hg, Pb, Sb, Zn and others (Volke-Sepulveda et al. 2003) in stream sediments of the SJLP drainage basin (Posada-Ayala 2011). These studies indicate that 50 % of the stream sediment samples are above the international guidelines (20 mg As Kg−1; WHO 2001). Moreover, PTEs have contaminated 50 km of arroyo sediments in the El Carrizal basin (Marmolejo-Rodriguez et al. 2011; Romero-Guadarrama 2011), and the 18 km adjacent to the mining district are the most contaminated. In the SJLP basin (Posada-Ayala 2011) along the San Antonio stream, the average total As content in sediment samples is 729 mg Kg−1, while the background level of As reported in sediments is 7.8 mg As Kg−1 (Marmolejo-Rodriguez et al. 2011; Posada-Ayala 2011; Romero-Guadarrama 2011), indicating this value is three times greater than the average in the crust (Wedepohl 1995).
These areas have reported As measurements in drinking water above the National (25 ng As mL−1; NOM-127-SSA 1994) and International (10 ng As mL−1; WHO 2001) maximum contaminant levels (MCL) for drinking water. Studies by the Mexican National Water Commission (CNA 2003) and Niparaja-Guardianes del Agua (2011)evaluated As levels in 42 and 80 wells respectively in the SJLP aquifer. CNA data, indicated that 40.5 % of the wells contained 0.35–10 ng As mL−1, 21.5 % contained 10.1–25 ng As mL−1, 31 % contained 25.1–200 ng As mL−1 and 7 % contained 200.1–2,270 ng As mL−1, indicating 38 % of the wells were above the National and 59.5 % of the wells were above the International MCL for drinking water. Previous studies (Smith et al. 1992; Celik et al. 2008) show that low levels of As may be carcinogenic, where the cancer risk for those ingesting drinking water containing levels of 2.5 ng As mL−1 (1.6 L day−1) is 1/1,000 persons, and at 50 ng As mL−1 (1.6 L day−1) is 21/1,000 persons.
Materials and methods
Study subjects
A cross-sectional study was conducted in 275 adults (66 % female, 34 % male) from seven small towns in the Baja California Sur peninsula of Mexico, which represented 4.8 % of the total population in the area. The localities were selected as they were the most densely populated zones and previous geochemical studies had been performed for water, sediment and mine waste in the area. The population of these towns in the SJLP and El Carrizal basins depended on the groundwater/aquifers within these hydrological basins and rain water which supplies these aquifers, due to contact with local rocks and mine waste that contain iAs minerals, such as arsenopyrite (FeAsS) and arsenolita (As2 O3), contaminates the groundwater.
This study was approved by the Institutional Review Boards of the Health Departments and Assistant Secretary of the state. Written (informed) consent was provided by all study participants, and urine samples were collected between August and October 2012. Participants were interviewed about their age, drinking water sources, current dietary habits, on-going/previous diseases and time of residence in the study area. Pregnant women, alcoholics and individuals with chronic or acute diseases of the urinary tract were excluded from the study.
Urine collection from participants
All urine samples were obtained at home under fasting conditions. First morning void urine samples were collected in disposable urine collection cups and delivered to the Health Center for immediate urinalysis. Samples were placed into three 15-ml polyethylene tube, frozen within 1–2 h of collection and stored at −20 °C at the CICIMAR laboratory.
Arsenic urinary analysis
One aliquot of each sample was transported on dry ice to Mexico City (CINVESTAV laboratory) for As speciation analysis, where iAs and its metabolites (MMA and DMA) were evaluated. Arsenical species were determined by hydride generation (HG)-cryotrapping (CT)- atomic absorption spectrometry (AAS) using a Perkin Elmer Analyst 400 equipped with a FIAS-200 flow injection atomic spectroscopy system and background corrector (Hernández-Zavala et al. 2008). This methodology analyses iAs, MMA and DMA concentrations in urine (expressed as ng As mL−1), and limits of detection (in urine) were 0.10, 0.15 and 0.6 ng mL−1 for iAs, MMA and DMA, respectively. The total concentration of As species in urine (tAs) was reported as the sum of the iAs (arsenite and arsenate), MMA(3+ and 5+) and DMA(3+ and 5+) concentrations. Replicate analyses of the standard reference material (SRM) 2,669 (NIST standard, quality control) showed less than an 8 % coefficient of variation.
Evaluation of arsenic exposure and metabolism
The concentration of tAs in the urine was used to estimate individual exposure to iAs. Urinary iAs (MMA and DMA) speciation was used as an indicator of As metabolism (Del Razo et al. 1997). The proportion of As species (%iAs, %MMA and %DMA) was calculated by dividing the concentration of each As species by the tAs in urine.
Statistical analyses
Preliminary analyses were performed to assess data quality, consistency and the distribution of the variables of interest. Continuous variables presented non-normal distributions, except age, %iAs, %MMA and %DMA, which were normally distributed. All continuous variables were reported as median and range, as well as mean ± the standard deviation, while frequencies or percentages were reported for categorical variables. Exposure was stratified into two categories, <35 ng mL−1 and >35 ng mL−1, where 35 ng mL−1 represented the Biological Exposure Index (BEI) or permissible limit for occupational As exposure (ACGIH 2010). Differences in species ratios between the Carrizal basin and SJLP basin were evaluated by the Mann–Whitney test. Spearman’s correlation coefficients (rS) were used to estimate associations between the relative proportion of As species and tAs. p values <0.05 were considered statistically significant. All statistical analyses were performed using Stata version 10 (StataCorp, College Station, TX, USA).
Results
Characteristics of the study population
The basic characteristics of the study population, including gender and age, are shown in Table 1. The average age of the participants was 44 ± 14 years, and 56–97 % were females (highest number from El Carrizal) and 2.5–44 % were males (highest number from Juan Domínguez Cota).
Urinary arsenic levels
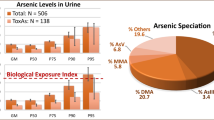
The As species concentrations and the tAs (iAs + MMA + DMA) levels in urine are shown in Table 2, where 33 % of samples indicated levels above the BEI. The percentages of people above the BEI were as follows: San Antonio −57.14 %, Los Planes −47.5 %, El Carrizal −40 %, Juan Domínguez Cota −32.79 %, Melitón Albañez −22.73 %, El Sargento −20.73 % and El Triunfo−12.3 % (Fig. 2). The urinary tAs range was 1.32–399 ng mL−1 and subjects with higher tAs concentrations were from San Antonio and Los Planes (median of 40.38 and 34.99 ng mL−1, respectively) followed by Juan Dominguez Cota, Melitón Albáñez, El Carrizal, El Triunfo and El Sargento (25.32, 24.37, 24.23, 24.15 and 21.53 ng mL−1, respectively).
The percentage of urine samples with the total As species concentration in urine above the Biological Exposure Index value of 35 ng mL−1. San Juan de los Planes Basin: San Antonio, Juan Domínguez Cota (JDC), Los Planes, El Sargento. El Carrizal Basin: El Triunfo, El Carrizal and Melitón Albáñez (MA)
The proportions of As species in the urine of each individual were also considered, where DMA was the predominant species (80.8 and 72.8 %, respectively) in the SJLP and El Carrizal basin samples, compared to MMA (9.6 and 10.46 %, respectively). Differences in urine %iAs speciation profiles were also different between basins and were greater in samples from the El Carrizal basin (17.5 vs 8.7 %) compared to the SJLP basin (Fig. 3).
The efficiency of the methylation process was also assessed by using the MMA/iAs and DMA/MMA ratios. The MMA/iAs ratios of the subjects in the SJLP basin were significantly higher than those of the El Carrizal basin subjects (p < 0.05), while no differences were seen between the DMA/MMA ratios (Table 2).
With respect to As speciation profiles, statistically significant positive correlations were found between the %MMA and urinary tAs concentrations in samples from both basins (Fig. 4). An inverse correlation was also found between %iAs and tAs concentrations for the El Carrizal basin (rS = −236; p = 0.015) and a marginal correlation (rS = 134; p = 0.069) for the SJLP basin.
Discussion
The present study found a high proportion of study participants (33 %) had excessive exposure to As (>BEI) based on tAs levels in urine. The BEI was used as a guideline or recommendation to control for potential workplace health hazards, and this value (35 ng mL−1) did not account for hydration status (i.e., for creatinine or specific gravity). First void urine was collected, and generally, first void urine was considered to be unaffected by urine dilution. Also chronic exposure to iAs and high urinary As levels have been linked to an increased creatinine concentration in urine (Basu et al. 2011). As associations between iAs exposure based on creatinine-adjusted urinary As measures can be higher than estimates based on urinary As levels not adjusted for creatinine (Chen et al. 2010), urinary concentrations were reported as ng As mL−1.
The subjects were primarily adult women from the SJLP (62 ± 7 %) and the El Carrizal basins (76 ± 19 %). Smokers made up 0–13 % (highest number from Melitón Albañez), and 8–55 % drank alcohol (highest value is from El Carrizal) (Table 1). The concentration of tAs in urine could be attributed to groundwater contamination due to geogenic and anthropogenic sources, specifically the release of iAs from the arsenopyrite minerals in the surrounding mountains and from the arsenolite in tailings and ash from the ignition ovens of abandoned gold mines (Carrillo 1996; Posada-Ayala 2011; Marmolejo-Rodriguez et al. 2011; Sánchez-Martínez et al. 2013a, b).
A HG-based method was used in this study to detect iAs and the methylated arsenicals (MMA and DMA) produced by iAs metabolism in human tissues. Urinary As concentrations were similar to those found in other areas (United States—Pellizzari and Clayton 2006; Zimapan, Mexico—Del Razo et al. 2011). Nevertheless, as a result, As species found in urine from the study subjects were associated exclusively with exposure to iAs and not from As species commonly found in seafood (arsenosugars, arsenobetaine or arsenocholine), which are not efficiently (or not at all) converted to hydrides during the HG step (Currier et al. 2013).
There are several studies showing an association between %MMA and increased risk for iAs-related disease in humans (Del Razo et al. 1997; Valenzuela et al. 2005; Steinmaus et al. 2006), which provide a body of evidence linking %MMA to iAs-related disease risks. The urinary MMA detected in this study included both the trivalent and pentavalent forms (3+ and 5+). An interesting finding in the present study was the increased percentage of MMA (p < 0.05) in urine, which was associated with increasing concentrations of urinary tAs in participants from both basins. The higher concentration of MMA (3+ and 5+) in the urine could represent higher levels of MMAIII, which was the more reactive and toxic intermediate metabolite of iAs methylation (compared to other As species). The urinary MMA3+ level may serve as an indicator to identify individuals with increased susceptibility to the toxic and cancer-promoting effects of arsenicosis (Valenzuela et al. 2005), and urinary MMA concentration could be considered an indirect marker of converted MMA3+.
The use of the MMA/iAs and DMA/MMA ratios to assess methylation merits further consideration, especially as iAs and MMA concentrations are generally present in urine in similar magnitudes, and any small change in the levels of these two species generates large differences in the MMA/iAs ratio. This was seen in samples from the El Carrizal basin, where iAs concentrations were higher than those from the SJLP basin, resulting in a large MMA/iAs range (Table 2).
Adverse effects of inorganic arsenic exposure
This study indicated a large portion of the total population from the Baja California Sur peninsula of Mexico is at risk due to chronic exposure to high concentrations of iAs. The adverse impact of iAs exposure on human health has been documented, as iAs is a potent human carcinogen (IARC 2004) linked to cancer in several organs including the bladder, lungs, skin, kidney and liver (ATSDR 2007), as well as hypertension, atherogenic effects (Chen et al. 1996) and diabetes (Del Razo et al. 2011). Further studies are needed to identify sources of iAs exposure to allow better intervention. As there are no easy solutions to high As contamination, addressing iAs contamination issues would require a variety of approaches from different fields of research.
Conclusions
Results of this study suggested residents from the Baja California Sur Peninsula were chronically exposed to high levels of iAs. Specifically, 33 % of subjects participating in this study had urinary As concentrations above the BEI. Thus, a systematic testing of drinking water, as well as sources of soil and air contamination, for the presence of As should be a top priority for the local government and public health authorities. Given the potential for adverse health effects, preventative actions should be implemented in these localities, and the drinking water, soil and air quality monitored and regulated. Also environment remediation should be addressed.
References
ACGIH, American Conference of Governmental Industrial Hygienists. (2010). Threshold limit values (TLVs) for chemical substances and physical agents and biological exposure indices (BEIs) 210 p.
ATSDR, Agency for Toxic Substances and Disease Registry. (2007). Toxicological profile for arsenic. Atlanta, GA: U.S Department of Health and Human Services, Public Health Service.
Basu, A., Mitra, S., Chung, J., Mazumder, D. N. G., Ghosh, N., Kalman, D., et al. (2011). Creatinine, diet, micronutrients, and arsenic methylation in West Bengal, India. Environmental Health Perspectives, 119, 1308–1313.
Bodénan, F., Baranger, P., Piantone, P., Lassin, A., Azaroual, M., Gaucher, E., et al. (2004). Arsenic behaviour in gold-ore mill tailings, Massif Central, France: Hydro geochemical study and investigation of in situ redox signatures. Applied Geochemistry, 19, 1785–1800.
Carrillo, A. (1996). Enviromental geochemistry of the San Antonio—El Triunfo mining area, southernmost Baja California Peninsula Mexico. PhD Thesis in Geology, Laramie Wyoming, 130 p.
Celik, I., Gallicchio, L., Boyd, K., Lam, T. K., Matanoski, G., Tao, X., et al. (2008). Arsenic in drinking water and lung cancer: A systematic review. Environmental Research, 108, 48–55.
Chen, Y., Ahsan, H., Slavkovich, V., Peltier, G. L., Gluskin, R. T., Parvez, F., et al. (2010). No association between arsenic exposure from drinking water and diabetes mellitus: A cross-sectional study in Bangladesh. Environmental Health Perspectives, 118, 1299–1305.
Chen, C. I., Chiou, H. Y., Chiang, M. H., Lin, L. J., & Tai, T. Y. (1996). Dose-response relationship between ischemic heart disease and long term arsenic exposure. Arteriosclerosis, Thrombosis, and Vascular Biology, 16, 504–510.
Cho, Y. M., Seo, S. C., Choi, S. H., Lee, S. K., Kim, K. H., Kim, H. J., et al. (2013). Association of arsenic levels in soils and water with urinary arsenic concentrations of residents in vicinity of closed metal mines. International Journal of Hygiene and Environmental Health, 216, 255–262.
Chun-Yu, R., Hung, K.-H., Chen, C.-J., & Froines, J. R. (2000). Arsenic methylation capacity and skin cancer. Cancer Epidemiology, Biomarkers and Prevention, 9, 1259–1262.
CNA. (2003). Comisión Nacional del Agua. Gerencia estatal en Baja California Sur. Estudio de caracterización de la intrusión salina en el acuífero de los Planes BCS. 525 p.
Coronado-González, J. A., Del Razo, L. M., García-Vargas, G., Sanmiguel-Salazar, F., & de la Escobedo Peña, J. (2007). Inorganic arsenic exposure and type 2 diabetes mellitus in Mexico. Environmental Research, 104, 383–389.
Crecelius, E. A. (1977). Changes in the chemical speciation of arsenic following ingestion by man. Environmental Health Perspectives, 19, 147–150.
Currier, J. M., Saunders, J., Ding, L., Bodnar, W., Cable, P., Matoušek, T., et al. (2013). Comparative oxidation state specific analysis of arsenic by high-performance liquid chromatography-inductively coupled plasma-mass spectrometry and hydride generation-cryotrapping-atomic absorption spectrometry. Journal Analytical Atomic Spectrometry, 28, 843–852.
Del Razo, L. M., García-Vargas, G., Valenzuela, O. L., Hernández-Castellanos, E., Sánchez-Peña, L. C., Currier, J. M., et al. (2011). Exposure to arsenic in drinking water is associated with increased prevalence of diabetes: A cross-sectional study in the Zimapan and Lagunera regions in Mexico. Environmental Health, 10(73), 1–11.
Del Razo, L. M., García-Vargas, G. G., Vargas, H., Albores, A., Gonsebatt, M. E., Montero, R., et al. (1997). Altered profile of urinary arsenic metabolites in adults with chronic arsenicism: A pilot study. Archives of Toxicology, 71(4), 211–217.
Heitland, P., & Köster, H. D. (2009). Comparison of different medical cases in urinary arsenic speciation by fast HPLC-ICP-MS. International Journal of Hygiene and Environmental Health, 212, 432–438.
Herbel, M. J., Switzer Blum, J., Hoeft, S. E., Cohen, S. M., Arnold, L. L., Lisak, J., et al. (2002). Dissimilatory arsenate reductase activity and arsenate –respiring bacteria in bovine rumen fluid, hamster feces and the termite hindgut. FEMS Microbiology Ecology, 41, 59–67.
Hernández-Zavala, A., Matoušek, T., Drobná, Z., Adair, B. M., Dĕdina, J., Thomas, D. J., et al. (2008). Speciation of arsenic in biological matrices by automated hydride generation–cryotrapping–atomic absorption spectrometry with multiple microflame quartz tubeatomizer (multiatomizer). Journal Analytical Atomic Spectrometry, 23, 342–351.
Hughes, M. F., Beck, B. D., Chen, Y., Lewis, A. S., & Thomas, D. J. (2011). Arsenic exposure and toxicology: A historical perspective. Toxicology Science, 123, 305–332.
IARC (International Agency for Research on Cancer). (2004). Some drinking-water disinfectants and contaminants, including arsenic. In IARC Monographs on the evaluation of carcinogenic risks to humans (Vol. 84, pp. 1–19). World Health Organization.
INEGI (Instituto Nacional de Estadística Geografía e Informática). (2010). Censo de población y vivienda 2010. Consulta interactiva de datos. http://cuentame.inegi.org.mx/poblacion/habitantes.aspx.
Krysiak, A., & Karczewska, A. (2007). Arsenic extractability in soils in the areas of former arsenic mining and smelting, SW Poland. Science of the Total Environment, 379, 190–200.
Lim, H. S., Lee, J.-S., Chon, H.-T., & Sager, M. (2008). Heavy metal contamination and health risk assessment in the vicinity of the abandoned songcheon Au–Ag mine in Korea. Journal of Geochemical Exploration, 96, 223–230.
Marmolejo-Rodriguez, A. J., Sánchez-Martínez, M. A., Romero-Guadarrama, J. A., Sánchez-Gonzalez, A., & Magallanes-Ordoñez, V. R. (2011). Migration of As, Hg, Pb, and Zn in arroyo sediments from a semiarid coastal system influenced by the abandoned gold mining district at El Triunfo, Baja California Sur, México. Journal of Environmental Monitoring, 13, 2182–2189.
Mazumder, G. (2008). Chronic arsenic toxicity and human health. Indian Journal of Medical Research, 128, 436–447.
Nava-Sánchez, E. H. (1992). Sedimentología de la Cuenca San Juan de Los Planes, Baja California Sur, México. Masters Thesis (CICIMAR-IPN) 166 pp (In Spanish).
Niparaja-Guardianes del Agua (2011). Concentración de Arsénico en agua de pozos de la cuenca hidrológica de San Juan de Los Planes. Datos del Servicio Geológico Mexicano, orden 10120. Date: 11-03-2010. (In Spanish).
NOM-127-SSA. (1994). Norma Oficial Mexicana. Salud ambiental, agua para uso y consumo humano. Límites permisibles de calidad y tratamientos a que debe someterse el agua para su potabilización. Diario Oficial de la Federación. Mexico. (In Spanish).
NRC (National Research Council). (1999). Arsenic in drinking water. Washington, DC: National Academy Press.
Pellizzari, E. D., & Clayton, C. A. (2006). Assessing the measurement precision of various arsenic forms and arsenic exposure in the national human exposure assessment survey (NHEXAS). Environmental Health Perspectives, 140(2), 220–227.
Posada-Ayala, I.H. (2011). Geoquímica ambiental del Distrito Minero San Antonio, sedimentos de arroyos de la Cuenca de San Juan de los Planes y plataforma continental de Bahia La Ventana, BCS, Mexico. Masters Thesis (CICIMAR-IPN) 210 pp. (In Spanish).
Robles, G.S. (1985). Estudio geográfico del Estado de Baja California Sur. Dirección de Cultura Gobierno de BCS, México. In: E.H. Nava-Sánchez (eds) (1992). Sedimentología de la Cuenca San Juan de Los Planes, Baja California Sur, México. Master Thesis (CICIMAR-IPN), 178p (In Spanish).
Romero-Guadarrama, J.A. (2011). Geoquímica de As, Hg, Pb y Zn y mineralogía en sedimentos superficiales de la cuanca de drenaje del distrito minero El Triunfo, BCS., México. Masters Thesis (CICIMAR-IPN) 98 pp (In Spanish).
Sánchez-Martínez, M. A., Marmolejo-Rodríguez, A. J., Gómez-Millán, R., Sánchez-González, A., Magallanes-Ordóñez, V. R., Romero-Guadarrama, J. A., et al. (2013a). Sediment accumulation of Ag, Cu, and Ni through a semi-arid basin as a by-product of the El Triunfo gold mine, Baja California Sur México. Journal of Iberian Geology, 39(1), 97–110.
Sánchez-Martínez, M. A., Marmolejo-Rodríguez, A. J., Magallanes-Ordóñez, V. R., & Sánchez-González, A. (2013b). Vertical accumulation of potential toxic elements in a semiarid system that is influenced by an abandoned gold mine. Estuarine Coastal and Shelf Sciences, 130, 42–53.
Smith, A. H., Hopenhayn-Rich, C., & Bates, M. N. (1992). Cancer risk from arsenic in drinking water. Environmental Health Perspectives, 97, 259–267.
Soleo, L., Lovreglio, P., Iavicoli, S., Antelmi, A., Drago, I., Basso, A., et al. (2008). Significance of urinary arsenic speciation in assessment of seafood ingestion as the main source of organic and inorganic arsenic in a population resident near a coastal area. Chemosphere, 73, 291–299.
Steinmaus, C., Bates, M. N., Yuan, Y., Kalman, D., Atallah, R., Rey, O. A., et al. (2006). Arsenic methylation and bladder cancer risk in case–control studies in Argentina and the United States. Journal Occupational Environmental Medicine, 48(5), 478–488.
Valenzuela, O. L., Borja-Aburto, V. H., García Vargas, G., Cruz-González, M., Garcia-Montalvo, E., Calderon-Aranda, E., et al. (2005). Urinary trivalent methylated arsenic species in a population chronically exposed to inorganic arsenic. Enviromental Health Perspectives, 113(3), 250–254.
Volke-Sepulveda, T., Solorzano-Ochoa, G., Rosas-Domínguez, A., Izumikawa, C., Aguilar, G.E., Velazco-Trejo, J.A., & Flores-Martínez, S. (2003). Remediación de sitios contaminados por metales provenientes de jales mineros en los distritos de El Triunfo—San Antonio y Santa Rosalia, Baja California Sur. Informe Técnico. Dirección de Investigación en Residuos y proyectos regionales. Centro de Investigación y capacitación ambiental. Instituto Nacional de Ecología. Technical Report. 1–37 (In Spanish).
Watson, W. A., Litovitz, T. L., Rodger, S. G. C., Klein-Schwartz, W., Youniss, J., Rose, S. R., et al. (2003). 2002 Annual report of the American association of poison control center toxic exposure surveillance system. The American Journal of Emergency Medicine, 21, 353–421.
Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica et Cosmochimica Acta, 59(7), 1217–1232.
WHO (World Health Organization) 2001. Arsenic in drinking water, http://www.who.int/inf-fs/en/fact210.html.
Acknowledgments
This study was supported by the Consejo Nacional de Ciencia y Tecnología—project No. 98710 (SIP 20101323 and SIP20131030). We gratefully thank the Health Chairman of La Paz County and the Health Center Directors of each town who organized talks to ask people to help in the study, and a special thanks to the participants that collaborated in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Colín-Torres, C.G., Murillo- Jiménez, J.M., Del Razo, L.M. et al. Urinary arsenic levels influenced by abandoned mine tailings in the Southernmost Baja California Peninsula, Mexico. Environ Geochem Health 36, 845–854 (2014). https://doi.org/10.1007/s10653-014-9603-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-014-9603-x