Abstract
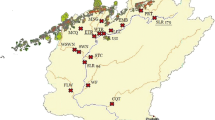
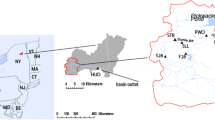
Mercury (Hg) is a toxic pollutant, widespread in northeastern US ecosystems. Resource managers’ efforts to develop fish consumption advisories for humans and to focus conservation efforts for fish-eating wildlife are hampered by spatial variability. Dragonfly larvae can serve as biosentinels for Hg given that they are widespread in freshwaters, long-lived, exhibit site fidelity, and bioaccumulate relatively high mercury concentrations, mostly as methylmercury (88% ± 11% MeHg in this study). We sampled lake water and dragonfly larvae in 74 northeastern US lakes that are part of the US EPA Long-Term Monitoring Network, including 45 lakes in New York, 43 of which are in the Adirondacks. Aqueous dissolved organic carbon (DOC) and total Hg (THg) were strongly related to MeHg in lake water. Dragonfly larvae total mercury ranged from 0.016–0.918 μg/g, dw across the study area; Adirondack lakes had the minimum and maximum concentrations. Aqueous MeHg and dragonfly THg were similar between the Adirondack and Northeast regions, but a majority of lakes within the highest quartile of dragonfly THg were in the Adirondacks. Using landscape, lake chemistry, and lake morphometry data, we evaluated relationships with MeHg in lake water and THg in dragonfly larvae. Lakewater DOC and lake volume were strong predictors for MeHg in water. Dragonfly THg Bioaccumulation Factors (BAFs, calculated as [dragonfly THg]:[aqueous MeHg]) increased as lake volume increased, suggesting that lake size influences Hg bioaccumulation or biomagnification. BAFs declined with increasing DOC, supporting a potential limiting effect for MeHg bioavailability with higher DOC.





Similar content being viewed by others
References
Allen EW, Prepas EE, Gabos S, Strachan WM, Zhang W (2005) Methyl mercury concentrations in macroinvertebrates and fish from burned and undisturbed lakes on the Boreal Plain. Can J Fish Aquat Sci 62:1963–1977
Bloom NS (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci 49(5):1010–1017
Bodaly RA, Fudge RJP (1999) Uptake of mercury by fish in an experimental boreal reservoir. Arch Environ Contam Toxicol 37(1):103–109
Branfireun BA, Roulet NT, Kelly CA, Rudd JWM (1999) In situ sulphate stimulation of mercury methylation in a boreal peatland: toward a link between acid rain and methylmercury contamination in remote environments. Glob Biogeochem Cycles 13(3):743–750
Braaten HFV, de Wit HA, Fjeld E, Rognerud S, Lydersen E, Larssen T (2014) Environmental factors influencing mercury speciation in Subarctic and Boreal lakes. Sci Total Environ 476–477:336–345
Bravo AG, Bouchet S, Tolu J, Björn E, Mateos-Rivera A, Bertilsson S (2017) Molecular composition of organic matter controls methylmercury formation in boreal lakes. Nat Commun 8:14255
Breiman L (2001) Random forests. Mach Learn 45(1):5–32
Broadley HJ, Cottingham KL, Baer NA, Weathers KC, Ewing HA, Chaves-Ulloa R, Chickering J, Wilson AM, Shrestha J, Chen CY (2019) Factors affecting MeHg bioaccumulation in stream biota: the role of dissolved organic carbon and diet. Ecotoxicology 28(8):949–963
Buckland-Nicks A, Hillier KN, Avery TS, O’Driscoll NJ (2014) Mercury bioaccumulation in dragonflies (Odonata: Anisoptera): examination of life stages and body regions. Environ Toxicol Chem 33:2047–2054. https://doi.org/10.1002/etc.2653
Chaves-Ulloa R, Taylor BW, Broadley HJ, Cottingham KL, Baer NA, Weathers KC, Ewing HA, Chen CY (2016) Dissolved organic carbon modulates mercury concentrations in insect subsidies from streams to terrestrial consumers. Ecol Applic 26:1771–1784
Chen CY, Stemberger RS, Klaue B, Blum JD, Pickhardt PC, Folt CL (2000) Accumulation of heavy metals in food web components across a gradient of lakes. Limnol Oceanogr 45(7):1525–1536
Chen CY, Driscoll CT, Kamman NC (2012) Mercury hotspots in freshwater ecosystems: drivers, processes, and pattern. In: Bank MS (ed.) Mercury in the environment: pattern and process. University of California Press, Berkeley, California, p 143–166
Chen CY, Folt CL (2005) High plankton biomass reduces mercury biomagnification. Environ Sci Technol 39:115–121
Chen CY, Stemberger RS, Kamman NC, Mayes BM, Folt CL (2005) Patterns of Hg bioaccumulation and transfer in aquatic food webs across multi-lake studies in the Northeast US. Ecotoxicology 14:135–147
Corbet PS (1999) Dragonflies: behavior and ecology of Odonata. Harley Books, Colchester, p 829
Creed IF, McKnight DM, Pellerin BA, Green MB, Bergamaschi BA, Aiken GR, Burns DA, Findlay SE, Shanley JB, Striegl RG, Aulenbach BT (2015) The river as a chemostat: fresh perspectives on dissolved organic matter flowing down the river continuum. Can J Fish Aquat Sci 72(8):1272–1285
DeWild J, Olson M, Olund S (2002) Determination of methylmercury by aqueous phase ethylation, followed by gas chromatographic separation with cold vapor atomic fluorescence detection. USGS Open File Report 01–445, Middleton, WI
Dittman JA, Driscoll CT (2009) Factors influencing changes in mercury concentrations in lake water and yellow perch (Perca flavescens) in Adirondack lakes. Biogeochemistry 93(3):179–196
Dittman JA, Shanley JB, Driscoll CT, Aiken GR, Chalmers AT, Towse JE, Selvendiran P (2010) Mercury dynamics in relation to dissolved organic carbon concentration and quality during high flow events in three northeastern U.S. streams. Water Resources Res 46(7)
Driscoll CT, Blette V, Yan C, Schofield CL, Munson R, Holsapple J (1995) The role of dissolved organic carbon in the chemistry and bioavailability of mercury in remote Adirondack lakes. Water Air Soil Pollut 80(1–4):499–508
Driscoll CT, Han Y-J, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK (2007) Mercury contamination in forest and freshwater ecosystems in the northeastern United States. BioScience 57(1):17–28
Eagles-Smith CA, Ackerman JT, Willacker JJ, Tate MT, Lutz MA, Fleck JA, Stewart AR, Wiener JG, Evers DC, Lepak JM, Davis JA (2016b) Spatial and temporal patterns of mercury concentrations in freshwater fish across the Western United States and Canada. Sci Tot Environ 568:1171–1184
Eagles-Smith CA, Herring G, Johnson B, Graw R (2016c) Conifer density within lake catchments predicts fish mercury concentrations in remote subalpine lakes. Environ Pollut 212:279–289
Eagles-Smith CA, Wiener JG, Eckley CS, Willacker JJ, Evers DC, Marvin-DiPasquale M, Obrist D, Fleck JA, Aiken GR, Lepak JM, Jackson AK (2016a) Mercury in western North America: a synthesis of environmental contamination, fluxes, bioaccumulation, and risk to fish and wildlife. Sci Tot Environ 568:1213–1226
Eagles-Smith CA, Suchanek TH, Colwell AE, Anderson NL (2008) Mercury trophic transfer in a eutrophic lake: the importance of habitat-specific foraging. Ecol Appl 18:196–212
Evers DC, Savoy LJ, DeSorbo CR, Yates DE, Hanson W, Taylor KM, Siegel LS, Cooley JH, Bank MS, Major A, Munney K (2008) Adverse effects from environmental mercury loads on breeding common loons. Ecotoxicology 17(2):69–81
Evers DC, Han Y-J, Driscoll CT, Kamman NC, Goodale MW, Lambert KF, Holsen TM, Chen CY, Clair TA, Butler T (2007) Biological mercury hotspots in the northeastern United States and southeastern Canada. Bioscience 57(1):29–43
Flanagan Pritz C, Eagles-Smith C, Krabbenhoft D (2014) Mercury in the national parks. George Wright Forum 31(2):168–180
Gavin AL, Nelson SJ, Klemmer AJ, Fernandez IJ, Strock KE, McDowell WH (2018) Acidification and climate linkages to increased dissolved organic carbon in high elevation lakes. Water Resour Res 54(8):5376–5393
Genuer R, Poggi JM, Tuleau-Malot C (2015) VSURF: an r package for variable selection using random forests. R J 7(2):19–33
Gorski PR, Armstrong DE, Hurley JP, Krabbenhoft DP (2008) Influence of natural dissolved organic carbon on the bioavailability of mercury to a freshwater alga. Environ Pollut 154(1):116–123
Grigal DF (2002) Inputs and outputs of mercury from terrestrial watersheds: a review. Environ Rev 10:1–39
Haines TA, May TW, Finlayson RT, Mierzykowski SE (2003) Factors affecting food chain transfer of mercury in the vicinity of the Nyanza Site, Sudbury River, Massachusetts. Environ Monit Assess 86:211–232. https://doi.org/10.1023/A:1024017329382
Hall BD, Aiken GR, Krabbenhoft DP, Marvin-DiPasquale M, Swarzenski CM (2008) Wetlands as principal zones of methylmercury production in southern Louisiana and the Gulf of Mexico region. Environ Pollut 154(1):124–134
Hammerschmidt CR, Fitzgerald WF (2006) Methylmercury in freshwater fish linked to atmospheric mercury deposition. Environ Sci Technol 40:7764–7770. https://doi.org/10.1021/es061480i
Hammerschmidt CR, Wiener JG, Frazier BE, Rada RG (1999) Methylmercury content of eggs in yellow perch related to maternal exposure in four Wisconsin lakes. Environ Sci Technol 33(7):999–1003
Haro RJ, Bailey SW, Northwick RM, Rolfhus KR, Sandheinrich MB, Wiener JG (2013) Burrowing dragonfly larvae as biosentinels of methylmercury in freshwater food webs. Environ Sci Technol 47:8148–8156. https://doi.org/10.1021/es401027m
Jackson B, Taylor V, Baker RA, Miller E (2009) Low-level mercury speciation in freshwaters by isotope dilution GC-ICP-MS. Environ Sci Technol 43(7):2463–2469
Jaeger B (2016) R2glmm: computes R squared for mixed (multilevel) models. R package version 0.1.2. https://cran.r-project.org/web/packages/r2glmm/r2glmm.pdf
Jeremiason JD, Reiser TK, Weitz RA, Berndt ME, Aiken GR (2016) Aeshnid dragonfly larvae as bioindicators of methylmercury contamination in aquatic systems impacted by elevated sulfate loading. Ecotoxicology 25(3):456–468
Jeremiason JD, Engstrom DR, Swain EB, Nater EA, Johnson BM, Almendinger JE, Monson BA, Kolka RK (2006) Sulfate addition increases methylmercury production in an experimental wetland. Environ Sci Technol 40(12):3800–3806
Johnson KB, Haines TA, Kahl JS, Norton SA, Amirbahman A, Sheehan KD (2007) Controls on mercury and methylmercury deposition for two watersheds in Acadia National Park, Maine. Environ Monit Assess 126(1–3):55–67
Jones TA, Chumchal MM, Drenner RW, Timmins GN, Nowlin WH (2013) Bottom-up nutrient and top-down fish impacts on insect-mediated mercury flux from aquatic ecosystems. Environ Tox Chem 32:612–618. https://doi.org/10.1002/etc.2079
Kahl JS, Norton SA, MacRae RK, Haines TA, Davis RB (1989) The influence of organic acidity on the chemistry of Maine surface waters. Water Air Soil Pollut 46:221–234
Kahl JS, Stoddard J, Haeuber R, Paulsen R, Birnbaum R, Deviney F, DeWalle D, Driscoll C, Herlihy A, Kellogg J, Murdoch P, Roy K, Sharpe W, Urquhart S, Webb R, Webster K (2004) Response of surface water chemistry to changes in acidic deposition: implications for future amendments to Clean Air Act. Environ Sci Technol 38:484A–490A
Kamman NC, Burgess NM, Driscoll CT, Simonin HA, Goodale W, Linehan J, Estabrook R, Hutcheson M, Major A, Scheuhammer AM, Scruton DA (2005) Mercury in freshwater fish of northeast North America–a geographic perspective based on fish tissue monitoring databases. Ecotoxicology 14(1–2):163–180
Kamman N, Driscoll CT, Estabrook B, Evers DC, Falmouth ME, Miller EK (2003) Biogeochemistry of mercury in Vermont and New Hampshire Lakes: an assessment of mercury in waters, sediments and biota of Vermont and New Hampshire Lakes. Comprehensive Final Project Report. US EPA, Waterbury, VT, p 110
Kamman NC, Lorey PM, Driscoll CT, Estabrook R, Major A, Pientka B, Glassford E (2004) Assessment of mercury in waters, sediments, and biota of New Hampshire and Vermont lakes, USA, sampled using a geographically randomized design. Environ Tox Chem 23(5):1172–1186
Klemmer AJ (2015) Unravelling the effects of multiple cross-ecosystem subsidies on food webs. PhD Dissertation, University of Canterbury. http://hdl.handle.net/10092/11861
Kolka RK, Nater EA, Grigal DF, Verry ES (1999) Atmospheric inputs of mercury and organic carbon into a forested upland/bog watershed. Water Air Soil Pollut 113(1–4):273–294
Loftin CS, Calhoun AJ, Nelson SJ, Elskus AA, Simon K (2012) Mercury bioaccumulation in wood frogs developing in seasonal pools. Northeastern Naturalist 19(4):579–601
Mason RP, Laporte J-M, Andres S (2000) Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish. Arch Environ Contam Toxicol 38:283–297. https://doi.org/10.1007/s002449910038
McHugh PA, McIntosh AR, Jellyman PG (2010) Dual influences of ecosystem size and disturbance on food chain length in streams. Ecol Lett 13(7):881–890
Miller EK, Chen C, Kamman N, Shanley J, Chalmers A, Jackson B, Taylor V, Smeltzer E, Stangel P, Shambaugh A (2012) Mercury in the pelagic food web of Lake Champlain. Ecotoxicology 21(3):705–718
Nelson SJ, Webber HM, Flanagan Pritz CM (2015) Citizen scientists study mercury in dragonfly larvae: Dragonfly larvae provide baseline data to evaluate mercury in parks nationwide; Natural Resource Report NPS/NRSS/ARD/NRR—2015/938. National Park Service, Fort Collins, Colorado
Nelson SJ, Eagles-Smith C, Pritz CF, Willacker J, Klemmer A, Blakesley A, Hess M (2018) Dragonfly mercury project: sampling guide for the collection of dragonfly larvae samples from national parks for mercury analysis. Project Protocol. https://www.nps.gov/articles/dragonfly-mercury-project.htm
Nelson SJ, Johnson KB, Weathers KC, Loftin CS, Fernandez IJ, Kahl JS, Krabbenhoft DP (2008) A comparison of winter mercury accumulation at forested and no-canopy sites measured with different snow sampling techniques. Appl Geochem 23(3):384–398
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2018) nlme: linear and nonlinear mixed effects models. R package version 3.1–137. https://cran.r-project.org/web/packages/nlme/nlme.pdf
Post DM, Pace ML, Hairston Jr NG (2000) Ecosystem size determines food-chain length in lakes. Nature 405(6790):1047
R Development Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Riva-Murray K, Chasar LC, Bradley PM, Burns DA, Brigham ME, Smith MJ, Abrahamsen TA (2011) Spatial patterns of mercury in macroinvertebrates and fishes from streams of two contrasting forested landscapes in the eastern United States. Ecotoxicology 20:1530–1542. https://doi.org/10.1007/s10646-011-0719-9
Sandheinrich MB, Wiener JG (2011) Methylmercury in freshwater fish: recent advances in assessing toxicity of environmentally relevant exposures. In: Beyer WN, Meador JP (eds) Environmental contaminants in biota: interpreting tissue concentrations, CRC Press, New York, NY, p 169–190
Schelker J, Burns DA, Weiler M, Laudon H (2011) Hydrological mobilization of mercury and dissolved organic carbon in a snow-dominated forested watershed: conceptualization and modeling. J Geophys Res 116:G01002. https://doi.org/10.1029/2010JG001330
Schuster PF, Shanley JB, Marvin-Dipasquale M, Reddy MM, Aiken GR, Roth DA, Taylor HE, Krabbenhoft DP, DeWild JF (2007) Mercury and Organic Carbon Dynamics During Runoff Episodes from a Northeastern USA Watershed. Water Air Soil Pollut 187(1–4):89–108
Shanley JB, Mast MA, Campbell DH, Aiken GR, Krabbenhoft DP, Hunt RJ, Walker JF, Schuster PF, Chalmers A, Aulenbach BT, Peters NE, Marvin-DiPasquale M, Clow DW, Shafer MM (2008) Comparison of total mercury and methylmercury cycling at five sites using the small watershed approach. Environ Pollut 154(1):143–154
Simonin HA, Loukmas JJ, Skinner LC, Roy KM (2008) Lake variability: key factors controlling mercury concentrations in New York State fish. Environ Pollut 154(1):107–115
Soltesz K (1996) Identification Key to Northeastern Anisoptera Larvae. University of Connecticut, CT
St. Louis VL, Rudd JWM, Kelly CA, Beaty KG, Bloom NS, Flett RJ (1994) The importance of wetlands as sources of methyl mercury to boreal forest ecosystems. Can J Fish Aquat Sci 51:1065–1076
Stemberger RS, Chen CY (1998) Fish tissue metals and zooplankton assemblages of northeastern US lakes. Can J Fish Aquat Sci 55(2):339–352
Stoddard JL, Urquhart NS, Newell AD, Kugler D (1996) The temporally integrated monitoring of ecosystems (TIME) project design: 2. Detection of regional acidification trends. Water Resour Res 32(8):2529–2538
Strock KE, Saros JE, Nelson SJ, Birkel SD, Kahl JS, McDowell WH (2016) Extreme weather years drive episodic changes in lake chemistry: implications for recovery from sulfate deposition and long-term trends in dissolved organic carbon. Biogeochemistry 127(2–3):353–365
Strock KE, Nelson SJ, Kahl JS, Saros JE, McDowell WH (2014) Decadal trends reveal recent acceleration in the rate of recovery from acidification in the northeastern US. Environ Sci Tech 48(9):4681–4689
Sullivan TJ, Driscoll CT, Beier CM, Burtraw D, Fernandez IJ, Galloway JN, Gay DA, Goodale CL, Likens GE, Lovett GM, Watmough SA (2018) Air pollution success stories in the United States: the value of long-term observations. Environ Sci Policy 84:69–73
Takimoto G, Spiller DA, Post DM (2008) Ecosystem size, but not disturbance, determines food‐chain length on islands of the Bahamas. Ecology 89(11):3001–3007
Tennessen KJ (2008) Odonata. In: Merritt RW, Cummins KW, Berg MB (eds). An introduction to the aquatic insects of North America, 4th edn. Kendall/Hunt Publishing Company, Dubuque, p 237–294
Tremblay, A (1999) Bioaccumulation of methylmercury in invertebrates from boreal hydroelectric reservoirs. In: Mercury in the biogeochemical cycle. Springer, Berlin, Heidelberg, p 193–214
Tremblay A, Lucotte M, Meili M, Cloutier L, Pichet P (1996) Total mercury and methylmercury contents of insects from boreal lakes: ecological, spatial and temporal patterns. Water Qual Res J Can 31(4):851–873
Tsui MT, Finlay JC (2011) Influence of dissolved organic carbon on methylmercury bioavailability across Minnesota stream ecosystems. Environ Sci Technol 45(14):5981–5987
Ward DM, Nislow KH, Chen CY, Folt CL (2010) Reduced trace element concentrations in fast-growing juvenile Atlantic Salmon in natural streams. Environ Sci Technol 44:3245–3251. https://doi.org/10.1021/es902639a
Watras CJ, Back RC, Halvorsen S, Hudson RJ, Morrison KA, Wente SP (1998) Bioaccumulation of mercury in pelagic freshwater food webs. Sci Total Environ 219(2–3):183–208
Wiener JG, Knights BC, Sandheinrich MB, Jeremiason JD, Brigham ME, Engstrom DR, Woodruff LG, Cannon WF, Balogh SJ (2006) Mercury in soils, lakes, and fish in Voyageurs National Park (Minnesota): importance of atmospheric deposition and ecosystem factors. Environ Sci Technol 40(20):6261–6268
Young HS, McCauley DJ, Dunbar RB, Hutson MS, Ter-Kuile AM, Dirzo R (2013) The roles of productivity and ecosystem size in determining food chain length in tropical terrestrial ecosystems. Ecology 94(3):692–701
Acknowledgements
Capable field crews made this project possible: A. Baumann, H. Roebuck Broadley, M. Prakash, C. Schmitt, K. Johnson, J. McKay, S. Edmonds, K. Warner. Dartmouth lab staff (B. Jackson, C. McCleery, H. Roebuck Broadley, K. Buckman) were instrumental in sample identification, processing, and analysis. Pam Hunt of NH Audubon assisted with dragonfly sample identification. David Krabbenhoft of the USGS-Wisconsin Water Science Center helped develop the project and provided input on an early draft of this manuscript. The Adirondack Lakes Survey Corporation sampled many Adirondack Lakes for water Hg and dragonfly larvae and provided logistical support for Maine-based crews sampling in the Adirondack region. K. Roy provided information about the Adirondack study lakes. A. Klemmer provided information regarding foodweb influence and discussion. Staff from state and tribal resource management agencies assisted with permitting and local contacts.
Funding
This project was supported by the Northeastern States Research Cooperative through funding made available by the USDA Forest Service. The US Environmental Protection Agency-US Geological Survey LTM/TIME project was funded by EPA ORD and EPA CAMD (IAG 06HQGR0143), processed through Grant/Cooperative Agreement G11AP20128 from the US Geological Survey. This research was also supported by the National Institutes of Health Grant P42 ESO7373 from the National Institute of Environmental Health Sciences. The Adirondack Lakes Survey Corporation, Adirondack Long Term Monitoring work was supported by the New York State Energy Research and Development Authority, the New York State Department of Environmental Conservation Division of Air Resources and the US EPA Clean Air Markets Division. This manuscript has not been subjected to agency review and no official endorsement by any agency should be inferred.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or vertebrate animals performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nelson, S.J., Chen, C.Y. & Kahl, J.S. Dragonfly larvae as biosentinels of Hg bioaccumulation in Northeastern and Adirondack lakes: relationships to abiotic factors. Ecotoxicology 29, 1659–1672 (2020). https://doi.org/10.1007/s10646-019-02149-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-019-02149-4