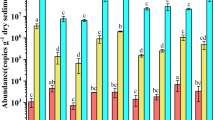
Abstract
Ecophysiological differences between ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA) enable them to adapt to different niches in complex freshwater wetland ecosystems. The community characters of AOA and AOB in the different niches in a freshwater wetland receiving municipal wastewater, as well as the physicochemical parameters of sediment/soil samples, were investigated in this study. AOA community structures varied and separated from each other among four different niches. Wetland vegetation including aquatic macrophytes and terrestrial plants affected the AOA community composition but less for AOB, whereas sediment depths might contribute to the AOB community shift. The diversity of AOA communities was higher than that of AOB across all four niches. Archaeal and bacterial amoA genes (encoding for the alpha-subunit of ammonia monooxygenases) were most diverse in the dry-land niche, indicating O2 availability might favor ammonia oxidation. The majority of AOA amoA sequences belonged to the Soil/sediment Cluster B in the freshwater wetland ecosystems, while the dominant AOB amoA sequences were affiliated with Nitrosospira-like cluster. In the Nitrosospira-like cluster, AOB amoA gene sequences affiliated with the uncultured ammonia-oxidizing beta-proteobacteria constituted the largest portion (99 %). Moreover, independent methods for phylogenetic tree analysis supported high parsimony bootstrap values. As a consequence, it is proposed that Nitrosospira-like amoA gene sequences recovered in this study represent a potentially novel cluster, grouping with the sequences from Gulf of Mexico deposited in the public databases.




Similar content being viewed by others
References
Adair KL, Schwartz E (2008) Evidence that ammonia-oxidizing archaea are more abundant than ammonia-oxidizing bacteria in semiarid soils of northern Arizona, USA. Microb Ecol 56:420–426. doi:10.1007/s00248-007-9360-9
Avrahami S, Conrad R (2003) Patterns of community change among ammonia oxidizers in meadow soils upon long-term incubation at different temperatures. Appl Environ Microbiol 69:6152–6164. doi:10.1128/aem.69.10.6152-6164.2003
Avrahami S, Conrad R, Braker G (2002) Effect of soil ammonium concentration on N2O release and on the community structure of ammonia oxidizers and denitrifiers. Appl Environ Microbiol 68:5685–5692. doi:10.1128/aem.68.11.5685-5692.2002
Beman JM, Francis CA (2006) Diversity of ammonia-oxidizing archaea and bacteria in the sediments of a hypernutrified subtropical estuary: Bahía del Tóbari, Mexico. Appl Environ Microbiol 72:7767–7777. doi:10.1128/aem.00946-06
Beman JM, Popp BN, Francis CA (2008) Molecular and biogeochemical evidence for ammonia oxidation by marine Crenarchaeota in the Gulf of California. ISME J 2:429–441
Briones AM, Okabe S, Umemiya Y, Ramsing N-B, Reichardt W, Okuyama H (2002) Influence of different cultivars on populations of ammonia-oxidizing bacteria in the root environment of rice. Appl Environ Microbiol 68:3067–3075. doi:10.1128/aem.68.6.3067-3075.2002
Bruns MA, Stephen JR, Kowalchuk GA, Prosser JI, Paul EA (1999) Comparative diversity of ammonia oxidizer 16S rRNA gene sequences in native, tilled, and successional soils. Appl Environ Microbiol 65:2994–3000
Cao H, Hong Y, Li M, Gu J-D (2012) Community shift of ammonia-oxidizing bacteria along an anthropogenic pollution gradient from the Pearl River Delta to the South China Sea. Appl Microbiol Biotechnol 94:247–259. doi:10.1007/s00253-011-3636-1
Christensen PB, Nielsen LP, Sørensen J, Revsbech NP (1990) Denitrification in nitrate-rich streams: diurnal and seasonal variation related to benthic oxygen metabolism. Limnol Oceanogr 35:640–651
Clément J-C, Pinay G, Marmonier P (2002) Seasonal dynamics of denitrification along topohydrosequences in three different riparian wetlands. J Environ Qual 31:1025–1037
Dang H, Zhang X, Sun J, Li T, Zhang Z, Yang G (2008) Diversity and spatial distribution of sediment ammonia-oxidizing crenarchaeota in response to estuarine and environmental gradients in the Changjiang Estuary and East China Sea. Microbiology 154:2084–2095. doi:10.1099/mic.0.2007/013581-0
Dionisi HM, Layton AC, Harms G, Gregory IR, Robinson KG, Sayler GS (2002) Quantification of Nitrosomonas oligotropha-like ammonia-oxidizing bacteria and Nitrospira spp. from full-scale wastewater treatment plants by competitive PCR. Appl Environ Microbiol 68:245–253
Dorador C, Busekow A, Vila I, Imhoff JF, Witzel K-P (2008) Molecular analysis of enrichment cultures of ammonia oxidizers from the Salar de Huasco, a high altitude saline wetland in northern Chile. Extremophiles 12:405–414
Erguder TH, Boon N, Wittebolle L, Marzorati M, Verstraete W (2009) Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol Rev 33:855–869. doi:10.1111/j.1574-6976.2009.00179.x
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Francis CA, Roberts KJ, Beman JM, Alyson ES, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci USA 102:14683–14688
Freney JR, Smith CJ, Mosier AR (1992) Effect of a new nitrification inhibitor (wax coated calcium carbide) on transformations and recovery of fertilizer nitrogen by irrigated wheat. Fertil Res 32:1–11
Geets J, Boon N, Verstraete W (2006) Strategies of aerobic ammonia-oxidizing bacteria for coping with nutrient and oxygen fluctuations. FEMS Microbiol Ecol 58:1–13
Herrmann M, Saunders AM, Schramm A (2008) Archaea dominate the ammonia-oxidizing community in the rhizosphere of the freshwater macrophyte Littorella uniflora. Appl Environ Microbiol 74:3279–3283. doi:10.1128/aem.02802-07
Herrmann M, Saunders AM, Schramm A (2009) Effect of lake trophic status and rooted macrophytes on community composition and abundance of ammonia-oxidizing prokaryotes in freshwater sediments. Appl Environ Microbiol 75:3127–3136. doi:10.1128/aem.02806-08
Hollibaugh JT, Bano N, Ducklow HW (2002) Widespread distribution in polar oceans of a 16S rRNA gene sequence with affinity to Nitrosospira-like ammonia-oxidizing bacteria. Appl Environ Microbiol 68:1478–1484
Hou P-F (2009) Status of Longfeng wetland and its protection recommendations. Heilongjiang Agr Sci 1:53–59
Ibekwe AM, Grieve CM, Lyon SR (2003) Characterization of microbial communities and composition in constructed dairy wetland wastewater effluent. Appl Environ Microbiol 69:5060–5069
Jiang H, Dong H, Yu B, Lv G, Deng S, Berzins N, Dai M (2009) Diversity and abundance of ammonia-oxidizing archaea and bacteria in Qinghai Lake, Northwestern China. Geomicrobiol J 26:199–211
Kim O-S, Junier P, Imhoff JF, Witzel K-P (2008) Comparative analysis of ammonia monooxygenase (amoA) genes in the water column and sediment-water interface of two lakes and the Baltic Sea. FEMS Microbiol Ecol 66:367–378
Könneke M, Bernhard AE, Torre JRdl, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543–546
Koops H-P, Pommerening-Röser A (2001) Distribution and ecophysiology of the nitrifying bacteria emphasizing cultured species. FEMS Microbiol Ecol 37:1–9
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55:485–529. doi:10.1146/annurev.micro.55.1.485
Kowalchuk GA, Stienstra AW, Heilig GHJ, Stephen JR, Woldendorp JW (2000) Molecular analysis of ammonia-oxidising bacteria in soil of successional grasslands of the Drentsche A (The Netherlands). FEMS Microbiol Ecol 31:207–215
Laanbroek HJ, Speksnijder AGCL (2008) Niche separation of ammonia-oxidizing bacteria across a tidal freshwater marsh. Environ Microbiol 10:3017–3025
Lozupone CA, Hamady M, Kelley ST, Knight R (2007) Quantitative and qualitative β diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol 73:1576–1585
Lüdemann H, Arth I, Liesack W (2000) Spatial changes in the bacterial community structure along a vertical oxygen gradient in flooded paddy soil cores. Appl Environ Microbiol 66:754–762
Mintie A, Heichen R, Cromack K Jr, Myrold D, Bottomley P (2003) Ammonia-oxidizing bacteria along meadow-to-forest transects in the Oregon Cascade Mountains. Appl Environ Microbiol 69:3129–3136
Moin NS, Nelson KA, Bush A, Bernhard AE (2009) Distribution and diversity of archaeal and bacterial ammonia oxidizers in salt marsh sediments. Appl Environ Microbiol 75:7461–7468
Mosier AC, Francis CA (2008) Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ Microbiol 10:3002–3016
Mullins TD, Britschgi TB, Krest RL, Giovannoni SJ (1995) Genetic comparisons reveal the same unknown bacterial lineages in Atlantic and Pacific bacterioplankton communities. Limnol Oceanogr 40:148–158
Nicolaisen MH, Risgaard-Petersen N, Revsbech NP, Reichardt W, Ramsing NB (2004) Nitrification-denitrification dynamics and community structure of ammonia oxidizing bacteria in a high yield irrigated Philippine rice field. FEMS Microbiol Ecol 49:359–369
Norton JM, Alzerreca JJ, Suwa Y, Klotz MG (2002) Diversity of ammonia monooxygenase operon in autotrophic ammonia-oxidizing bacteria. Arch Microbiol 177:139–149
Ottosen LDM, Risgaard-Petersen N, Nielsen LP (1999) Direct and indirect measurements of nitrification and denitrification in the rhizosphere of aquatic macrophytes. Aquat Microb Ecol 19:81–91
Park H-D, Wells GF, Bae H, Criddle CS, Francis CA (2006) Occurrence of ammonia-oxidizing archaea in wastewater treatment plant bioreactors. Appl Environ Microbiol 72:5643–5647. doi:10.1128/aem.00402-06
Pett-Ridge J, Petersen DG, Nuccio E, Firestone MK (2013) Influence of oxic/anoxic fluctuations on ammonia oxidizers and nitrification potential in a wet tropical soil. FEMS Microbiol Ecol 85:179–194. doi:10.1111/1574-6941.12111
Pitcher A, Villanueva L, Hopmans EC, Schouten S, Reichart GJ, Sinninghe Damsté JS (2011) Niche segregation of ammonia-oxidizing archaea and anammox bacteria in the Arabian Sea oxygen minimum zone. ISME J 5:1896–1904
Pommerening-Röser A, Rath G, Koops HP (1996) Phylogenetic diversity within the genus Nitrosomonas. Syst Appl Microbiol 19:344–351
Prosser JI, Nicol GW (2008) Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ Microbiol 10:2931–2941
Purkhold U, Pommerening-Röser A, Juretschko S, Schmid MC, Koops H-P, Wagner M (2000) Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: implications for molecular diversity surveys. Appl Environ Microbiol 66:5368–5382. doi:10.1128/aem.66.12.5368-5382.2000
Purkhold U, Wagner M, Timmermann G, Pommerening-Röser A, Koops H-P (2003) 16S rRNA and amoA-based phylogeny of 12 novel betaproteobacterial ammonia-oxidizing isolates: extension of the dataset and proposal of a new lineage within the nitrosomonads. Int J Syst Evol Microbiol 53:1485–1494
Reigstad LJ, Richter A, Daims H, Urich T, Schwark L, Schleper C (2008) Nitrification in terrestrial hot springs of Iceland and Kamchatka. FEMS Microbiol Ecol 64:167–174
Riis T, Sand-Jensen K (1998) Development of vegetation and environmental conditions in an oligotrophic Danish lake over 40 years. Freshwat Biol 40:123–134
Ruiz-Rueda O, Hallin S, Baneras L (2009) Structure and function of denitrifying and nitrifying bacterial communities in relation to the plant species in a constructed wetland. FEMS Microbiol Ecol 67:308–319
Santoro AE, Casciotti KL (2011) Enrichment and characterization of ammonia-oxidizing archaea from the open ocean: phylogeny, physiology and stable isotope fractionation. ISME J 5:1796–1808. doi:10.1038/ismej.2011.58
Schloss PD, Handelsman J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71:1501–1506. doi:10.1128/aem.71.3.1501-1506.2005
Schloss PD et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Schramm A, de Beer D, Wagner M, Amann R (1998) Identification and activities in situ of Nitrosospiraand nitrospira spp. as dominant populations in a nitrifying fluidized bed reactor. Appl Environ Microbiol 64:3480–3485
Stehr G, Böttcher B, Dittberner P, Rath G, Koops H-P (1995) The ammonia-oxidizing nitrifying population of the River Elbe estuary. FEMS Microbiol Ecol 17:177–186
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Taylor AE, Zeglin LH, Wanzek TA, Myrold DD, Bottomley PJ (2012) Dynamics of ammonia-oxidizing archaea and bacteria populations and contributions to soil nitrification potentials. ISME J 6:2024–2032
Tourna M et al (2011) Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci USA 108:8420–8425. doi:10.1073/pnas.1013488108
Treusch AH, Leininger S, Kletzin A, Schuster SC, Klenk HP, Schleper C (2005) Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ Microbiol 7:1985–1995
Venter JC et al (2004) Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66–74. doi:10.1126/science.1093857
Verhoeven JTA, Meuleman AFM (1999) Wetlands for wastewater treatment: opportunities and limitations. Ecol Eng 12:5–12. doi:10.1016/s0925-8574(98)00050-0
Wagner M, Rath G, Amann R, Koops H-P, Schleifer K-H (1995) In situ identification of ammonia-oxidizing bacteria. Syst Appl Microbiol 18:251–264
Walker CB et al (2010) Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA 107:8818–8823. doi:10.1073/pnas.0913533107
Wang Y-F, Gu J-D (2013) Higher diversity of ammonia/ammonium-oxidizing prokaryotes in constructed freshwater wetland than natural coastal marine wetland. Appl Microbiol Biotechnol 97:7015–7033. doi:10.1007/s00253-012-4430-4
Wang Y-F, Gu J-D (2014) Effects of allylthiourea, salinity and pH on ammonia/ammonium-oxidizing prokaryotes in mangrove sediment incubated in laboratory microcosms. Appl Microbiol Biotechnol 98:3257–3274. doi:10.1007/s00253-013-5399-3
Wang Y, Ke X, Wu L, Lu Y (2009) Community composition of ammonia-oxidizing bacteria and archaea in rice field soil as affected by nitrogen fertilization. Syst Appl Microbiol 32:27–36
Wang J, Dong H, Wang W, Gu J-D (2013a) Reverse-transcriptional gene expression of anammox and ammonia-oxidizing archaea and bacteria in soybean and rice paddy soils of Northeast China. Appl Microbiol Biotechnol. doi:10.1007/s00253-013-5242-x
Wang J, Wang W, Gu J-D (2013b) Community structure and abundance of ammonia-oxidizing archaea and bacteria after conversion from soybean to rice paddy in albic soils of Northeast China. Appl Microbiol Biotechnol. doi:10.1007/s00253-013-5213-2
Wang Y-F, Feng Y-Y, Ma X, Gu J-D (2013c) Seasonal dynamics of ammonia/ammonium-oxidizing prokaryotes in oxic and anoxic wetland sediments of subtropical coastal mangrove. Appl Microbiol Biotechnol 97:7919–7934. doi:10.1007/s00253-012-4510-5
Wang Y-F, Li X-Y, Gu J-D (2014a) Differential responses of ammonia/ammonium-oxidizing prokaryotes in mangrove sediment to amendment of acetate and leaf litter. Appl Microbiol Biotechnol 98:3165–3180. doi:10.1007/s00253-013-5318-7
Wang Y, Zhu G, Song L, Wang S, Yin C (2014b) Manure fertilization alters the population of ammonia-oxidizing bacteria rather than ammonia-oxidizing archaea in a paddy soil. J Basic Microbiol 54:190–197. doi:10.1002/jobm.201200671
Wells GF, Park H-D, Yeung C-H, Eggleston B, Francis CA, Criddle CS (2009) Ammonia-oxidizing communities in a highly aerated full-scale activated sludge bioreactor: betaproteobacterial dynamics and low relative abundance of Crenarchaea. Environ Microbiol 11:2310–2328
Winogradsky S (1890) Investigations on nitrifying organisms. Ann Inst Pasteur 4:213–321
Wuchter C et al (2006) Archaeal nitrification in the ocean. Proc Natl Acad Sci USA 103:12317–12322
Xu C, Zhang G (2009) Biodiversities of Daqing wetland and countermeasures for protection. Chinese Agr Sci Bull 25:215–219
You J, Das A, Dolan EM, Hu Z (2009) Ammonia-oxidizing archaea involved in nitrogen removal. Water Res 43:1801–1809
Zhang L-M, Hu H-W, Shen J-P, He J-Z (2012) Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J 6:1032–1045
Zhou Z, Shi X, Zheng Y, Qin Z, Xie D, Li Z, Guo T (2014) Abundance and community structure of ammonia-oxidizing bacteria and archaea in purple soil under long-term fertilization. Europ J Soil Biol 60:24–33
Zuber G, Goldsmith MR, Beratan DN, Wipf P (2005) Assignment of the absolute configuration of [n]-ladderanes by TD-DFT optical rotation calculations. Chirality 17:507–510
Acknowledgments
This research was supported by a studentship from the Graduate School of The University of Hong Kong (KHL), additional financial support of this project was from Environmental Toxicology Education and Research Fund of this laboratory, and National Natural Science Foundation of China (41003031, 41273109, 51378208) and Shanghai Rising-Star Program (12QA1400800), and Fok Ying Tung Education Foundation (141077) (HL). We would like to thank Ms. Jessie Lai and Kelly Lau for support in chemical analysis and Dr. Jing Wang and Mr. Hongshuang Jiao for assistance in field sampling.
Conflict of interest
The authors have no conflict of interest in research results report here.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kwok-Ho Lee and Yong-Feng Wang were contributed to this work equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, KH., Wang, YF., Li, H. et al. Niche specificity of ammonia-oxidizing archaeal and bacterial communities in a freshwater wetland receiving municipal wastewater in Daqing, Northeast China. Ecotoxicology 23, 2081–2091 (2014). https://doi.org/10.1007/s10646-014-1334-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1334-3