Abstract
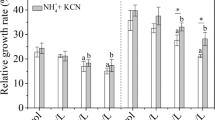
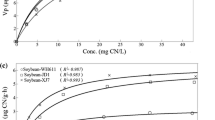
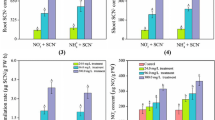
A study was conducted to investigate the contribution of β-cyanoalanine synthase (CAS) to the botanical metabolism of free cyanide and iron cyanides. Seedlings of rice (Oryza sativa L. cv. XZX 45) were grown hydroponically and then amended with free cyanide (KCN) or ferri-cyanide [K3Fe(CN)6] into the growth media. Total cyanide, free cyanide, and Fe3+/Fe2+ in aqueous solution were analyzed to identify the speciation of K3Fe(CN)6. Activity of CAS in different parts of the rice seedlings was also assayed in vivo and results indicated that dissociation of K3Fe(CN)6 to free cyanide in solution was negligible. Almost all of the applied KCN was removed by rice seedlings and the metabolic rates were concentration dependent. Phyto-transport of K3Fe(CN)6 was apparent, but appreciable amounts of cyanide were recovered in plant tissues. The metabolic rates of K3Fe(CN)6 were also positively correlated to the concentrations supplied. Rice seedlings exposed to KCN showed a considerable increase in the CAS activity and roots had higher CAS activity than shoots, indicating that CAS plays an important role in the botanical assimilation of KCN. However, no measurable change of CAS activity in different parts of rice seedlings exposed to K3Fe(CN)6 was detected, suggesting that K3Fe(CN)6 is likely metabolized by rice directly through an unknown pathway rather than the β-cyanoalanine pathway.










Similar content being viewed by others
References
Castric PA, Farnden KJF, Conn EE (1972) Cyanide metabolism in higher plants. V. The formation of asparagine from β-cyanoalanine. Arch Biochem Biophys 152:62–69
Dzombak DA, Ghosh RS, Young TC (2005) Physical–chemical properties and reactivity of cyanide in water and soil. In: Dzombak DA, Ghosh RS, Wong-Chong GW (eds) Cyanide in water and soil: chemistry, risk, and management. CRC, Boca Raton, pp 58–92
Ebbs SD, Bushey J, Poston S, Kosma D, Samiotakis M, Dzombak D (2003) Transport and metabolism of free cyanide and iron cyanide complexes by willow. Plant Cell Environ 26:1467–1478
Ebbs SD, Piccinin RC, Goodger JQD, Kolev SD, Woodrow IE, Baker AJM (2008) Transport of ferrocyanide by two eucalypt species and sorghum. Int J Phytoremediation 10:343–357
Ebbs SD, Kosma D, Nielson EH, Machingura M, Baker AJM, Woodrow IE (2010) Nitrogen supply and cyanide concentration influence the enrichment of nitrogen from cyanide in wheat (Triticum aestivum L.) and sorghum (Sorghum bicolor L.). Plant Cell Environ 33:1152–1160
Federico R, Giartosio CE (1983) A transplasmamembrane electron transport system in maize. Plant Physiol 73:182–184
Ghosh RS, Dzombak DA, Luthy RG, Nakles DV (1999) Subsurface fate and transport of cyanide species at a manufactured-gas plant site. Water Environ Res 71:1205–1216
Ghosh RS, Nakles DV, Murarka P, Neuhauser EF (2004) Cyanide speciation in soil and groundwater at manufactured gas plant (MGP) sites. Environ Eng Sci 21:752–767
Gibbs MM (1979) A simple method for the rapid determination of iron in natural waters. Water Res 13:295–297
Goudey JS, Tittle FL, Spencer MS (1989) A role for ethylene in the metabolism of cyanide by higher plants. Plant Physiol 89:1306–1310
Greenberg AE, Clesceri LSE, Eaton AD (1992) Standard methods for the examination of water and wastewater, 18rd edn. American Water Works Association. Water Pollution Control Federation, Washington, pp 366–368
Hendrickson HR, Conn EE (1969) Cyanide metabolism in higher plants. IV. Purification and properties of the beta-cyanoalanine synthase of blue lupine. J Biol Chem 244:2632–2640
Korte F, Spiteller M, Coulston F (2000) The cyanide leaching gold recovery process is a non-sustainable technology with unacceptable impacts on ecosystems and humans: The disaster in Romania. Ecotoxicol Environ Saf 46:241–245
Larsen M, Trapp S (2006) Uptake of iron cyanide complexes into willow trees. Environ Sci Technol 40:1956–1961
Larsen M, Ucisik A, Trapp S (2005) Uptake, metabolism, accumulation and toxicity of cyanide in willow trees. Environ Sci Technol 39:2135–2142
Liang WS (2003) Drought stress increases both cyanogenesis and beta-cyanoalanine synthase activity in tobacco. Plant Sci 165:109–1115
Machingura M, Ebbs S (2010) Increased beta-cyanoalanine synthase and asparaginase activity in nitrogen-deprived wheat exposed to cyanide. J Plant Nutr Soil Sci 173:808–810
Manning K (1988) Detoxification of cyanide by plants and hormone action. In: Ciba Foundation (ed) Cyanide compounds in biology. John Wiley & Sons, Chichester, pp 92–110
Mansfeldt T, Leyer H, Barmettler K, Kretzschmar R (2004) Cyanide leaching from soil developed from coking plant purifier waste as influenced by citrate. Vadose Zone J 3:471–479
Maruyama A, Saito K, Ishizawam K (2001) β-cyanoalanine synthase and cysteine synthase from potato: molecular cloning, biochemical characterization, and spatial and hormonal regulation. Plant Mol Biol 46:749–760
Meeussen JCL, Keizer MG, de Haan FAM (1992) Chemical stability and decomposition rate of iron cyanide complexes in soil solutions. Environ Sci Technol 26:511–516
Meeussen JCL, van Riemsdijk WH, van der Zee SEATM (1995) Transport of complexed cyanide in soil. Geoderma 67:73–85
Meyer T, Burow M, Bauer M, Papenbrock J (2003) Arabidopsis sulfurtransferases: investigation of their function during senescence and in cyanide detoxification. Planta 217:1–10
Miller JM, Conn EE (1980) Metabolism of hydrogen cyanide by higher plants. Plant Physiol 65:1199–1202
Mino Y, Ishida T, Ota N, Inoue M, Nomoto K, Takemoto T, Tanaka H, Sugiura Y (1983) Mugineic acid-iron (III) complex and its structurally analogous cobalt (III) complex: characterization and implication for absorption and transport of iron in gramineous plants. J Am Chem Soc 105:4611–4676
Mudder T, Botz M (2001) A guide to cyanide. Mining Environ Manag 9:8–12
Peiser GD, Wang TT, Hoffman NE, Yang SF, Walsh CT (1984) Formation of cyanide from carbon 1 of 1-aminocyclopropane-1-carbonxylic acid during its conversion to ethylene. Proc Natl Acad Sci USA 81:3059–3063
Rennert T, Mansfeldt T (2002) Sorption of iron-cyanide complexes on goethite in the presence of sulfate and desorption with phosphate and chloride. J Environ Qual 31:745–751
Römheld V, Marschner H (1986) Evidence for a specific uptake system for iron phytosiderophore in roots of grasses. Plant Physiol 80:175–180
Sachs L (1992) Angewandte Statistik. Springer, Berlin
Samiotakis M, Ebbs SD (2004) Possible evidence for transport of an iron cyanide complex by plants. Environ Pollut 127:169–173
Trapp S, Zambrano KC, Kusk KO, Karlson U (2000) A phytotoxicity test using transpiration of willows. Arch Environ Contam Toxicol 39:154–160
Yu XZ, Gu JD (2009) Uptake, accumulation and metabolic response of ferri-cyanide in weeping willows. J Environ Monit 11:145–152
Yu XZ, Gu JD (2010) Effect of Temperature on removal of iron cyanides from solutions by maize plants. Environ Sci Pollut Res 17:106–114
Yu XZ, Peng XY, Wang GL (2011) Photo induced dissociation of ferri and ferro cyanide in hydroponic solutions. Int J Environ Sci Technol 8:853–862
Acknowledgment
This work was financially supported by The National Science Foundation of China (NSFC: 40971256).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, XZ., Lu, PC. & Yu, Z. On the role of β-cyanoalanine synthase (CAS) in metabolism of free cyanide and ferri-cyanide by rice seedlings. Ecotoxicology 21, 548–556 (2012). https://doi.org/10.1007/s10646-011-0815-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0815-x