Abstract
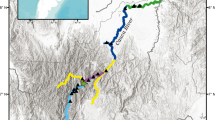
Pollution is one of the main concerns in marine ecosystems nowadays. Environmental anthropogenic-mediated toxicants may affect genetic diversity both at the individual and ecosystem levels and may also alter the genetic structure of populations. This study examined the temporal pattern of genetic diversity among populations of the benthic bivalve Ensis siliqua in two locations of Galicia, following the Prestige oil spillage. On November 13, 2002 the oil tanker Prestige sank at 240 km from Galician coast and 63,000 tonnes of heavy fuel were released to the marine environment. E. siliqua samples were sampled between 2001 and 2006. Genetic variation was assessed by means of Random Amplification of Polymorphic DNA (RAPD). A significant decrease in genetic diversity was observed for the 2006 samples. Nei’s genetic distance, fixation index (PhiPT), and PCA values also supported differences in the 2006 samples. We hypothesize that the temporal genetic variation observed in E. siliqua populations is due to a strong effect of genetic drift caused by a reduction in population size and that the indirect effects of the Prestige spill possibly caused this reduction.



Similar content being viewed by others
References
Abele D, Brey T, Philipp E (2009) Bivalve models of aging and the determination of molluscan lifespans. J Exp Ger. doi: 10.1016/j.exger.2009.02.012
Alfonsi CY, Nusetti O, Perez J (1995) Heterozygosity and metabolic efficiency in the scallop Euvola ziczac (Linneaus, 1758). J Shellfish Res 14:389–393
Aranishi F, Okimoto T (2004) Genetic relationship between cultured populations of Pacific oyster revealed by RAPD analysis. J Appl Genet 45:435–443
Arias A, Fernández-Moreno M, Fernández-Tajes J, Gaspar MB, Méndez J (2010) Strong genetic differentiation among east Atlantic populations of the sword razor shell (Ensis siliqua) assessed with mtDNA and RAPD markers. Helgol Mar Res. doi: 10.1007/s10152-010-0203-6
Avise JC (2000) Phylogeography, the history and formation of species. Harvard University Press, Cambridge
Beaumont AR, Gosling EM, Beveridge CM, Budd MD, Burnell GM (1985) Studies on heterozygosity and size in the scallop, Pecten maximus. In: Gibbs PE (ed) Proceedings of the 19th European marine biology symposium. Cambridge University Press, Cambridge, pp 443–454
Belfiore NM, Anderson SL (2001) Effects of contaminants on genetic patterns in aquatic organisms: a review. Mutat Res 489:97–122
Bickham JW, Sandhu S, Hebert PDN, Chikhi L, Athwal R (2000) Effects of chemical contaminants on genetic diversity in natural populations: implications for biomonitoring and ecotoxicology. Mutat Res 463:33–51
Bortz J, Lienert G, Boehnke K (2000) Verteilungsfreie Methoden in der Biostatistik. Springer, Berlin
Bricelj VM, Krause MK (1992) Resource allocation and population genetics of the bay scallop, Argopecten irradians irradians: effects of age and allozyme heterozygosity on reproductive output. Mar Biol 113:253–261
Brokordt K, Leiva N, Jeno K, Martínez G, Winkler F (2009) Effect of allozyme heterozygosity on basal and induced levels of heat shock protein (Hsp70), in juvenile Concholepas concholepas (Mollusca). J Exp Mar Biol Ecol 370:18–26
Calderón I, Palacín C, Turon X (2009) Microsatellite markers reveal shallow genetic differentiation between cohorts of the common sea urchin Paracentrotus lividus (Lamarck) in northwest Mediterranean. Mol Ecol 18:3036–3049
Chung PP, Hyne RV, Mann RM, O. Ballard JW (2010) Temporal and geogrpaphical variation in the amphipod Melita plumulosa (Crustacea: Melitidae): link of a localized change in haplotype frequencies to a chemical spill. Chemosphere. doi: 10.1016/j.chemosphere.2010.10.043
Da Costa F, Martínez-Patiño D, Ojea J, Novoa S (2010) Larval rearing and spat production of the razor clam Ensis siliqua (Bivalvia: Pharidae). J Shell Res 29:347–351
Darriba S, San Juan F, Guerra A (2005) Gametogenic cycle of Ensis siliqua (Linnaeus, 1758) in the ría de Corcubión, northwestern Spain. J Moll Stud 71:47–51
De Wolf H, Backeljau T, Verhagen R (1998) Congruence between allozyme and RAPD data in assessing macrogeographical genetic variation in the periwinkle Littorina striata (Mollusca, Gastropoda). Heredity 81:486–492
De Wolf H, Blust R, Backeljau T (2004a) The use of RAPD in ecotoxicology. Mutat Res 566:249–262
De Wolf H, Blust R, Backeljau T (2004b) The population genetic structure of Littorina littorea (Mollusca, Gastropoda) along a pollution gradient in the Scheldt estuary (The Netherlands) using RAPD analysis. Sci Total Environ 325:59–69
Diz AP, Presa P (2009) The genetic diversity pattern of Mytilus galloprovincialis in Galician Rias (NW Iberian estuaries). Aquaculture 287:278–285
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Fahy E, Gaffney J (2001) Growth statistics of an exploited razor clam (Ensis siliqua) bed at Gormanstown, Co Meath, Ireland. Hydrobiologia 465:139–151
Fahy E, Alcantara ML, Norman M, Browne R, Roantree V, Pfeiffer N (2002) Mortalities of Ensis arcuatus (Jeffreys) (Solenacea) in western Ireland. J Shell Res 21:29–32
Fernández-Tajes J, Gaspar M, Martínez-Patiño D, McDonough N, Roberts D, González-Tizón A, Martínez-Lage A, Méndez J (2007) Genetic variation of the razor clam Ensis siliqua (Jeffreys 1875) along the European coast based on random amplified polymorphic DNA markers. Aquac Res 38:1205–1212
Figueras A, Lora-Tamayo E, Tintoré J, Pérez F, Albaigés J, Sánchez F, Murado AG, Rodríguez EE, Hernán R (2005) Las lecciones de la catastrophe del Prestige. Consejo superior de Investigaciones Científicas, Madrid
Gaffney PM (1990) Enzyme heterozygosity, growth rate and viability in Mytilus edulis, another look. Evolution 44:204–209
Gaspar MB, Monteiro CC (1998) Reproductive cycles of the razor clam Ensis siliqua and the clam Venus striatula off Vilamoura, Southern Portugal. J Mar Biol Assoc UK 78:1247–1258
Gaspar MB, Richardson CA, Monteiro CC (1994) The effects of dredging on shell formation in the razor clam Ensis siliqua from Barrinha, southern Portugal. J Mar Biol Assoc UK 74:927–938
Hedgecock D (1994) Does variance in reproductive success limit effective population sizes of marine organisms). In: Beaumont AR (ed) Genetics and evolution of aquatic organisms. Chapman and Hall, London, pp 122–134
Joaquim S, Pereira J, Leitao A, Matías D, Chaves R, Guedes-Pinto H, Chícharo L, Gaspar M (2010) Genetic diversity of two Portuguese populations of the pullet carpet shell Venerupis senegalensis, based on RAPD markers: contribution to a sustainable restocking program. Helgol Mar Res 64:289–295
Johnston EL, Roberts DA (2009) Contaminants reduce the richness and evenness of marine communities: a review and meta-analysis. Environ Pollut 157:1745–1752
Koehn RK, Shumway SE (1982) A genetic/physiological explanation for differential growth rate among individuals of the American oyster, Crassostrea virginica (Gmelin). Mar Biol Lett 3:35–42
Körpe DA, Aras S (2011) Evaluation of copper-induced stress on eggplant (Solanum melongena L.) seedlings at the molecular and populations levels by use of various biomarkers. Mutat Res 19:29–34
Labarta U, Fernández-Reiriz MJ, Garrido JL, Babarro JMF, Bayona JM, Albaigés J (2005) Response of mussel recruits to pollution from the Prestige oil spill along the Galicia coast. A biochemical approach. Mar Ecol Prog Ser 302:135–145
Laffon B, Rábade T, Pásaro E, Méndez J (2006) Monitoring of the impact of Prestige oil spill on Mytilus galloprovincialis from Galician coast. Environ Int 32:342–348
Li Ma X, Cowles DL, Carter RL (2000) Effect of pollution on genetic diversity in the bay mussel Mytilus galloprovincialis and the acorn barnacle Balanus glandul. Mar Environ Res 50:559–563
Liu W, Yang YS, Zhou Q, Xie L, Li P, Sun T (2007) Impact assessment of cadmium contamination on rice (Oryza sativa L.) seedlings at molecular and population levels using multiple biomarkers. Chemosphere 67:1155–1163
Lynch M, Milligan BG (1994) Analysis of population genetic structure with RAPD markers. Mol Ecol 3:91–99
Marigómez I, Soto M, Cancio I, Orbea A, Garmendia L, Cajaraville MP (2006) Cell and tissue biomarkers in mussel, and histopathology in hake and anchovy from Bay of Biscay after the Prestige oil spill (Monitoring Campaign 2003). Mar Pollut Bull 53:287–304
Matson CW, Lambert MM, McDonald TJ, Autenrieth RL, Donnelly KC, Islamzadeh A, Politov DL, Bickham JW (2006) Evolutionary toxicology: population-level effects of chronic contaminant exposure on the marsh frogs (Rana ridibunda) of Azerbaijan. Environ Health Perspect 114:547–552
Miller MP (1997) Tools for Population Genetic Analyses (TFPGA) 1.3: a Windows Program for the analysis of allozyme and molecular population genetic data. Computer software distributed by author
Monteiro C, Gaspar M (1993) Bivalves do litoral oceânico algarvio: breve noticia sobre a situaçâo actual dos Principais bancos (Julho 1993). In: Relatório Tecnico Cientifico INIP, Lisboa, 65, p 19
Nadig SG, Lee KL, Adams SM (1998) Evaluating alterations of genetic diversity in sunfish populations exposed to contaminants using RAPD assay. Aquat Toxicol 43:163–178
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nony PA, Schnellmann RG (2001) Interactions between collagen IV and collagen-binding integrins in renal repair after sublethal injury. Mol Pharmacol 60:1226–1234
Palumbi SR (2004) Marine reserves and ocean neighbourhoods: the spatial scale of marine populations and their management. Ann Rev Environ Res 29:31–68
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Peteiro LG, Bábarro JMF, Labarta U, Fernández-Reiriz MJ (2006) Growth of Mytilus galloprovincialis after the Prestige oil spill. ICES J Mar Sci 63:1005–1013
Peteiro LG, Labarta U, Fernández-Reiriz MJ (2007) Variability in biochemical components of the mussel (Mytilus galloprovincialis) cultured after Prestige oil spill. Comp Biochem Physiol C 145:588–594
Piñeira J, Quesada H, Rolán-Alvarez E, Caballero A (2008) Genetic impact of the Prestige oil spill in wild populations of a poor disperse marine snail from intertidal rocky shores. Mar Poll Bull 56:270–281
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Raymond M, Rousset F (1995) An exact test for population differentiation. Evolution 49:1280–1283
Ross K, Cooper N, Bidwell JR, Elder J (2002) Genetic diversity and metal tolerance of two marine species: a comparison between populations from contaminated and reference sites. Mar Pollut Bull 44:671–679
Sokal RR, Rohlf FL (1995) Biometry, 3rd edn. WH Freeman and Company, New York
Street GT, Lotufo GR, Montagna PA, Fleeger JW (1998) Reduced genetic diversity in a meiobenthic copepod exposed to a xenobiotic. J Exp Mar Biol Ecol 222:93–111
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. http://www.R-project.org
Theodorakis CW, Bickham JW (2004) Molecular characterization of contaminant-indicative RAPD markers. Ecotoxicology 13:303–309
Theodorakis CW, Bickham JW, Lamb T (2001) Integration of genotoxicity and population genetic analyses in kangaroo rats (Dipodomys merriami) exposed to radionuclide contamination at the Nevada test site, USA. Environ Toxicol Chem 20:317–326
Varela MA, Martínez-Lage A, Gonzalez-Tizon AM (2009) Temporal genetic variation of microsatellite markers in the razor clam Ensis arcuatus (Bivalvia: Pharidae). J Mar Biol Assoc UK 89:1703–1707
Von Cosel R (1990) An introduction to the razor shells (Bivalvia: Solenacea). In: Morton B (ed) The Bivalvia proceedings of a memorial symposium in honour of Sir Charles Maurice Yongue. Hong Kong University Press, Hong Kong, pp 283–305
Acknowledgments
This work was funded by a PGIDIT03 RMA10301PR, and PGIDIT03 PX10302PR grants from Galicia Government and by PFSHARE and TIMES grants from Community Initiative Programme INTERREG-IIIB “Atlantic Area”. We are grateful to Mr. Jose García Gil for his technical assistance and to two anonymous reviewers for their valuable comments in the text.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Fernández-Tajes and A. Arias-Pérez have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Fernández-Tajes, J., Arias-Pérez, A., Fernández-Moreno, M. et al. Sharp decrease of genetic variation in two Spanish localities of razor clam Ensis siliqua: natural fluctuation or Prestige oil spill effects?. Ecotoxicology 21, 225–233 (2012). https://doi.org/10.1007/s10646-011-0781-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0781-3