Abstract
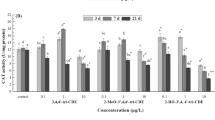
The importance of endocrine disrupting chemicals and their effects on fish has been documented in recent years. However, little is known about whether the estrogenic compound 17β estradiol (E2) causes oxidative stress in the hepatic tissue of fish. Therefore, this work tested the hypothesis that E2 might cause oxidative stress in the Japanese sea bass Lateolabrax japonicus liver. To test this hypothesis, its effects on reactive oxygen species (ROS) production, DNA damage, antioxidants and biotransformation enzyme were investigated in two different size groups (fingerling and juvenile groups) following 30 days exposure. Results showed that there was a good relationship between the E2 exposure concentration, plasma E2 level and ROS generation. In addition ROS production correlated negatively with 7-ethoxyresorufin-O-deethylase activity and positively with DNA damage and lipid peroxidation (LPO). Antioxidant enzymes such as superoxide dismutase and catalase did not show any significant relation with ROS, LPO and DNA damage. In contrast, glutathione mediated enzymes showed a good relationship with the above parameters suggesting that the glutathione system in fish might be responsible for protection against the impact of E2 and also indicating a possible adaptive response during exposure periods. In addition, it was observed that fingerling was more susceptible to E2 exposure than juvenile fish. The present study provided strong evidence that the ROS level increased significantly in the liver of E2 exposed fish, and that ROS might serve as a biomarker to indicate estrogen contamination.



Similar content being viewed by others
References
Ahmad I, Maria VL, Pacheco M, Santos MA (2009) Juvenile sea bass (Dicentrarchus labrax L.) enzymatic and non-enzymatic antioxidant responses following 17beta-estradiol exposure. Ecotoxicology 18:974–982
Arukwe A, Forlin L, Goksoyr A (1997) Xenobiotic and steroid biotransformation enzymes in Atlantic salmon (Salmo salar) liver treated with an estrogenic compound, 4-nonylphenol. Environ Toxicol Chem 16:2576–2583
Arukwe A, Kullman SW, Hinton DE (2001) Differential biomarker gene and protein expressions in nonylphenol and estradiol-17 beta treated juvenile rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol Part C 129:1–10
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burke MD, Mayer RT (1974) Ethoxyresorufin: direct fluorimetric assay of a microsomal O-dealkylation which is preferentially inducible by 3-methylcholanthrene. Drug Metab Dispos 2:583–588
Carrera EP, Garcia-Lopez A, del Rio MDM, Martinez-Rodriguez G, Sole M, Mancera JM (2007) Effects of 17 beta-estradiol and 4-nonylphenol on osmoregulation and hepatic enzymes in gilthead sea bream (Sparus auratus). Comp Biochem Physiol Part C 145:210–217
Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D (2000) Estrogens as endogenous genotoxic agents—DNA adducts and mutations. J Natl Cancer Inst Monogr 27:75–93
Cavalieri EL, Devanesan P, Bosland MC, Badawi AF, Rogan EG (2002) Catechol estrogen metabolites and conjugates in different regions of the prostate of noble rats treated with 4-hydroxyestradiol: implications for estrogen-induced initiation of prostate cancer. Carcinogenesis 23:329–333
Delaporte E, Renton KW (1997) Cytochrome P4501A1 and cytochrome P4501A2 are down regulated at both transcriptional and post-transcriptional levels by conditions resulting in interferon-alpha/beta induction. Life Sci 60:787–796
Devasagayam TP, Tarachand U (1987) Decreased lipid peroxidation in the rat kidney during gestation. Biochem Biophys Res Commun 145:134–138
Driver AS, Kodavanti PR, Mundy WR (2000) Age-related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol 22:175–181
Elskus AA (2004) Estradiol and estriol suppress CYP1A expression in rainbow trout primary hepatocytes. Mar Environ Res 58:463–467
Felty Q, Xiong WC, Sun D, Sarkar S, Singh KP, Parkash J, Roy D (2005) Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry 44:6900–6909
Giulio RTD, Washburn PC, Wenning RJ, Winston GW, Jewell CS (1989) Biochemical responses in aquatic animals: a review of determinants of oxidative stress. Environ Toxicol Chem 8:1103–1123
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hawkins SA, Billiard SM, Tabash SP, Brown RS, Hodson PV (2002) Altering cytochrome P4501A activity affects polycyclic aromatic hydrocarbon metabolism and toxicity in rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 21:1845–1853
Janssen YM, Van Houten B, Borm PJ, Mossman BT (1993) Cell and tissue responses to oxidative damage. Lab Invest 69:261–274
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42:656–666
Maria VL, Ahmad I, Santos MA (2008) Juvenile sea bass (Dicentrarchus labrax L.) DNA strand breaks and lipid peroxidation response following 17beta-estradiol two mode of exposures. Environ Int 34:23–29
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Mates JM, Perez-Gomez C, Nunez de Castro I (1999) Antioxidant enzymes and human diseases. Clin Biochem 32:595–603
Mather-Mihaich E, Di Giulio RT (1986) Antioxidant enzyme activities and malondialdehyde, glutathione and methemoglobin concentrations in channel catfish exposed to DEF and n-butyl mercaptan. Comp Biochem Physiol C 85:427–432
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochem Biophys Acta 582:67–78
Patel MM, Bhat HK (2004) Differential oxidant potential of carcinogenic and weakly carcinogenic estrogens: Involvement of metabolic activation and cytochrome P450. J Biochem Mol Toxicol 18:37–42
Rempel MA, Wang Y, Armstrong J, Schlenk D (2008) Uptake of estradiol from sediment by hornyhead turbot (Pleuronichthys verticalis) and effects on oxidative DNA damage in male gonads. Mar Environ Res 66:111–112
Rempel-Hester MA, Hong H, Wang Y, Deng X, Armstrong J, Gully J, Schlenk D (2009) Site-specific effects of 17beta-estradiol in hornyhead turbot (Pleuronichthys verticalis) collected from a wastewater outfall and reference location. Environ Res 109:552–558
Risso-de Faverney C, Lafaurie M, Girard JP, Rahmani R (2000) The nitroxide stable radical tempo prevents metal-induced inhibition of CYP1A1 expression and induction. Toxicol Lett 111:219–227
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Seacat AM, Kuppusamy P, Zweier JL, Yager JD (1997) ESR identification of free radicals formed from the oxidation of catechol estrogens by Cu +2 . Arch Biochem Biophys 347:45–52
Shugart LR (1988) Quantitation of chemically induced damage to DNA of aquatic organisms by alkaline unwinding assay. Aquat Toxicol 13:43–52
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Sole M, Porte C, Barcelo D (2000) Vitellogenin induction and other biochemical responses in carp, Cyprinus carpio, after experimental injection with 17 alpha-ethynylestradiol. Arch Environ Contam Toxicol 38:494–500
Sumpter JP, Johnson AC (2005) Lessons from endocrine disruption and their application to other issues concerning trace organics in the aquatic environment. Environ Sci Technol 39:4321–4332
Teles M, Gravato C, Pacheco M, Santos MA (2004) Juvenile sea bass biotransformation, genotoxic and endocrine responses to beta-naphthoflavone, 4-nonylphenol and 17 beta-estradiol individual and combined exposures. Chemosphere 57:147–158
Teles M, Pacheco M, Santos MA (2005) Sparus aurata L. liver EROD and GST activities, plasma cortisol, lactate, glucose and erythrocytic nuclear anomalies following short-term exposure either to 17beta-estradiol (E2) or E2 combined with 4-nonylphenol. Sci Total Environ 336:57–69
Teles M, Pacheco M, Santos MA (2006) Biotransformation, stress and genotoxic effects of 17beta-estradiol in juvenile sea bass (Dicentrarchus labrax L.). Environ Int 32:470–477
Thilagam H, Gopalakrishnan S, Bo J, Wang KJ (2009) Effect of 17beta-estradiol on the immunocompetence of Japanese sea bass (Lateolabrax japonicus). Environ Toxicol Chem 28:1722–1731
Thomas-Jones E, Thorpe K, Harrison N, Thomas G, Morris C, Hutchinson T, Woodhead S, Tyler C (2003) Dynamics of estrogen biomarker responses in rainbow trout exposed to 17beta-estradiol and 17alpha-ethinylestradiol. Environ Toxicol Chem 22:3001–3008
Vaccaro E, Meucci V, Intorre L, Soldani G, Di Bello D, Longo V, Gervasi PG, Pretti C (2005) Effects of 17beta-estradiol, 4-nonylphenol and PCB 126 on the estrogenic activity and phase 1 and 2 biotransformation enzymes in male sea bass (Dicentrarchus labrax). Aquat Toxicol 75:293–305
van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Wang MY, Liehr JG (1995) Lipid hydroperoxide-induced endogenous DNA adducts in hamsters: possible mechanism of lipid hydroperoxide-mediated carcinogenesis. Arch Biochem Biophys 316:38–46
Watson DE, Menard L, Stegeman JJ, Di Giulio RT (1995) Aminoanthracene is a mechanism-based inactivator of CYP1A in channel catfish hepatic tissue. Toxicol Appl Pharmacol 135:208–215
Wellejus A, Bornholdt J, Vogel UB, Risom L, Wiger R, Loft S (2004) Cell-specific oxidative DNA damage induced by estrogen in rat testicular cells in vitro. Toxicol Lett 150:317–323
Zhang X, Yang F, Xu Y, Liao T, Song S, Wang J (2008) Induction of hepatic enzymes and oxidative stress in Chinese rare minnow (Gobiocypris rarus) exposed to waterborne hexabromocyclododecane (HBCDD). Aquat Toxicol 86:4–11
Acknowledgments
This work was supported by the Minjiang Scholar Program to K-J. Wang (2009) and a grant (2007AA091406) from the National High Technology Research and Development Program of China (863 Program). We thank Prof. John. P. Giesy, Dept. Veterinary Biomedical Sciences, University of Saskatchewan, Canada for his assistance and comments in the statistical analysis of the manuscript. Professor John Hodgkiss is thanked for his assistance with English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thilagam, H., Gopalakrishnan, S., Qu, HD. et al. 17β estradiol induced ROS generation, DNA damage and enzymatic responses in the hepatic tissue of Japanese sea bass. Ecotoxicology 19, 1258–1267 (2010). https://doi.org/10.1007/s10646-010-0510-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0510-3