Abstract
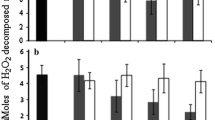
The bioaccumulation and harmful effects of microcystins (MCs) and the activity of peroxidase (POD) and superoxide dismutase (SOD) were examined in the apple (Malus pumila) exposed in vitro with the crude extract of toxic cyanobacterial blooms from Dianchi Lake in southwestern China. The results showed that the growth and proliferation of M. pumila shoots in vitro decreased markedly after exposure to microcystins above 0.3 μg/ml. Recovered microcystins determined by enzyme-linked immunosorbent assay (ELISA) in M. pumila shoot cultures increased with exposure time and concentration. After 14 days exposure to the concentration of 3 μg/ml microcystins, M. pumila shoot cultures accumulated microcystins up to a concentration of 510.23 ± 141.10 ng MC-LR equiv/g FW (fresh weight), equivalent to an accumulation rate of 36.45 ng/g day. POD activity was significantly increased after 7 days exposure to 3 μg/ml microcystins. After 14 days of exposure, microcystins caused POD to increase significantly at the concentration of 0.3 and 3 μg/ml. The activity of SOD was not affected by microcystins at concentrations up to 3 μg/ml on 7 days. After 14 days exposure to microcystins, SOD activity increased significantly at the concentration of 0.3 and 3 μg/ml in M. pumila shoot cultures.




Similar content being viewed by others
References
Abe T, Lawson T, Weyers JDB, Codd GA (1996) Microcystin-LR inhibits photosynthesis of Phaseolus vulgaris primary leaves: implications for current spray irrigation practice. New Phytol 133:651–658
Aranda-Rodriguez R, Kubwabo C, Benoit FM (2003) Extraction of 15 microcystins and nodularin using immunoaffinity columns. Toxicon 42:587–599
Bell SG, Codd GA (1994) Cyanobacterial toxins and human health. Rev Med Microbiol 5:869–872
Brzuzan P, Woźny M, Ciesielski S, Łuczyński MK, Góra M, Kuźmiński H, Dobosz S (2009) Microcystin-LR induced apoptosis and mRNA expression of p53 and cdkn1a in liver of whitefish (Coregonus lavaretus L.). Toxicon 54:170–183
Carmichael WW, Falconer IR (1993) Diseases related to freshwater blue-green algal toxins, and control measures. In: Falconer IR (ed) Algal toxins in seafood and drinking water. Academic Press, New York, pp 187–209
Cazenave J, Bistoni MA, Pesce SF, Wunderlin DA (2006) Differential detoxification and antioxidant response in diverse organs of Corydoras paleatus experimentally exposed to microcystin-RR. Aquat Toxicol 76:1–12
Chen JZ, Song LR, Dai J, Gan NQ, Liu ZL (2004) Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidase of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon 43:393–400
Codd GA, Bell SG, Brooks WP (1989) Cyanobacterial toxins in water. Water Sci Technol 21:1–13
Codd GA, Metcalf JS, Beattie KA (1999) Retention of Microcystis aeruginosa and microcystin by salad lettuce (Lactuca sativa) after spray irrigation with water containing cyanobacteria. Toxicon 37:1181–1185
Cousins IT, Bealing DJ, James HA, Sutton A (1996) Biodegradation of microcystin-LR by indigenous mixed bacterial populations. Water Res 30:481–485
Dawson RM (1998) The toxicology of microcystins. Toxicon 36:953–962
Ding WX, Shen HM, Ong CN (2001) Critical role of reactive oxygen species formation in microcystin-induced cytoskeleton disruption in primary cultured hepatocytes. J Toxicol Environ Health A 64:507–519
Hamvas MM, Mathe C, Molnar E, Vasa G, Grigorsky I, Borbely G (2002) Microcystin-LR alters the growth, anthanocyanin content and single-stranded DNase enzyme activities in Sinapis alba L. seedlings. Aquat Toxicol 61:1–9
Harada KI (1996) Chemistry and detection of microcysitns. In: Watanabe MF, Harada KI, Carmichael WW, Fujiki H (eds) Toxic Microcystis. CRC Press, Boca Raton, pp 103–148
Huang WM, Xing W, Li DH, Liu YD (2008) Microcystin-RR induced apoptosis in tobacco BY-2 suspension cells is mediated by reactive oxygen species and mitochondrial permeability transition pore status. Toxicol In Vitro 22:328–337
Järvenpää S, Lundberg-Niinistö C, Spoof L, Sjövall O, Tyystjärvi E, Meriluoto J (2007) Effects of microcystins on broccoli and mustard, and analysis of accumulated toxin by liquid chromatography—mass spectrometry. Toxicon 49:865–874
Jochimsen EM, Carmichael WW, An JS, Cardo DM, Cookson ST, Holmes CE, Antunes MB, de Melo Filho DA, Lyra TM, Barreto VS, Azevedo SM, Jarvis WR (1998) Liver failure and death after exposure to microcystins at a haemodialysis center in Brazil. New Engl J Med 338:873–878
Jones GJ, Orr PT (1994) Release and degradation of microcystin following algicide treatment of a Microcystis aeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res 28:871–876
Kós P, Gorzó G, Surányi G, Borbély G (1995) Simple and efficient method for isolation and measurement of cyanobacterial hepatotoxins by plant tests (Sinapis alba L.). Anal Biochem 225:49–53
Kujbida P, Hatanaka E, Campa A, Curi R, Farsky SHP, Pinto E (2008) Analysis of chemokines and reactive oxygen species formation by rat and human neutrophils induced by microcystin-LA, -YR and -LR. Toxicon 51:1274–1280
Kurki-Helasmo K, Meriluoto J (1998) Microcystin uptake inhibits growth and protein phosphatase activity in Mustard (Sinapis alba L.) seedlings. Toxicon 36:1921–1926
Lawton LA, Edwards C, Beattie KA, Pleasance S, Dear GJ, Codd GA (1995) Isolation and characterisation of microcystins from laboratory cultures and environmental samples of Microcystis aeruginosa and from an associated animal toxicosis. Nat Toxins 3:50–57
Lowry OH, Rosebrough NH, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA (1990) Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett 264:187–192
Máthé C, Beyer D, Erdődi F, Serfőző Z, Székvölgyi L, Vasas G, M-Hamvas M, Jámbrik K, Gonda S, Kiss A, Szigeti ZM, Surányi G (2009) Microcystin-LR induces abnormal root development by altering microtubule organization in tissue-cultured common reed (Phragmites australis) plantlets. Aquat Toxicol 92:122–130
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
McElhiney J, Lawton LA, Leifert C (2001) Investigations into the inhibitory effects of microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon 39:1411–1420
Mitrovic SM, Allis O, Furey A, James KJ (2005) Bioaccumulation and harmful effects of microcystin-LR in the aquatic plants Lemna minor and Wolffia arrhiza and the filamentous alga Chladophora fracta. Ecotox Environ Safe 61:345–352
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Oudra B, Loudiki M, Sbiyyaa B, Martins R, Vasconcelos V, Namikoshi N (2001) Isolation, characterization and quantification of microcystins (heptapeptides hepatotoxins) in Microcystis aeruginosa dominated bloom of Lalla Takerkoust lakereservoir (Morocco). Toxicon 39:1375–1381
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:570–577
Pflugmacher S (2004) Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat Toxicol 70:169–178
Pflugmacher S, Wiegand C, Beattie KA, Codd GA, Steinberg CEW (1998) Uptake of the cyanobacterial hepatotoxin microcystin-LR by aquatic macrophytes. J Appl Bot 72:228–232
Prieto A, Jos Á, Pichardo S, Moreno I, Cameán AM (2006) Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis sp.). Aquat Toxicol 77:314–321
Rao PVL, Bhattacharaya R, Das Gupta S (1994) Isolation, culture and toxicity of cyanobacterium (blue-green alga) Microcystis aeruginosa from freshwater source in India. B Environ Contam Tox 52:878–885
Rao PVL, Bhattacharaya R, Parida MM, Jana AM, Bhaskar ASB (1998) Freshwater cyanobacterium Microcystis aeruginosa (UTEX 2385) induced DNA damage in vivo and in vitro. Environ Toxicol Phar 5:1–6
Siegel G, MacKintosh C, Stitt M (1990) Sucrose-phosphate synthase is dephosphorylated by protein phosphotase 2A in spinach leaves: evidence from the effects of okadaic acid and microcystin. FEBS Lett 270:198–202
Ueno Y, Nagata S, Tsutsumi T, Hasegawa A, Watanabe MF, Park HD, Chen GC, Chen G, Yu SZ (1996) Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis 17:1317–1321
Yin LY, Huang JQ, Huang WM, Li DH, Wang GH, Liu YD (2005) Microcystin- RR-induced accumulation of reactive oxygen species and alteration of antioxidant systems in tobacco BY-2 cells. Toxicon 39:1411–1420
Žegura B, Sedmak B, Filipič M (2003) Microcystin-LR induces oxidative DNA damage in human hepatoma cell line HepG2. Toxicon 41:41–48
Acknowledgments
This research was funded by the National “863” High Science and Technology Project of China (AA-64-10-30) and Major Science and Technology Specific Project of Zhejiang Province (2008C12009). We would like to thank Dr. Nanqin Gan for her assistance in the determination of microcystins.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., Dai, J., Zhang, H. et al. Bioaccumulation of microcystin and its oxidative stress in the apple (Malus pumila). Ecotoxicology 19, 796–803 (2010). https://doi.org/10.1007/s10646-009-0456-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-009-0456-5