Abstract
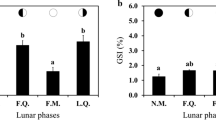
Identifying the hormonal signature of gonad maturity and spawning seasonality is essential for upgrading the forecast of recruitment, environmental impacts, and captive maturation. The study evaluated the pattern of gonad development and associated plasma levels of sex steroids and thyroid hormones in both sexes to identify gonadal stage markers and reproductive seasonality in an Indian shad, Tenualosa ilisha. Various stages depicted the cellular organization of ovarian and testicular developments. The ovarian histology revealed hilsa as group synchronous total spawners. The seasonal distribution of distinct maturity stages indicated unsynchronized gonad development within the population. Hilsa exhibited a differential preference of environmental cues in spring and late autumn spawning. However, peak spawning activity took place at similar photoperiod and temperature. The appearance of post-ovulatory follicles (POFs) and late spermiating testicular lobules (LS) marked active spawning in different sexes. The gonadosomatic index (IG) showed temporal, stage-specific variations with a positive correlation with plasma 11-KT (11-ketotestosterone), estradiol (E2) in females or with 17α, 20β-dihydroxy-4-pregnene-3-one (DHP) in males, although the relationship altered temporally for each variable. The positively correlated average plasma 11-KT and E2 values with the number of pre- and post-vitellogenic females serve as hormonal signatures for identifying initiation and completion of gonad growth, respectively. Higher circulating levels of 11-KT compared to T during annual gonad cycles indicate 11-KT as the predominant androgen that controls spermatogenesis in this species. Plasma DHP showed a minor peak in March and a significant elevation in October, synchronized with active spawning in females and males marked as a hormonal signature of spawning irrespective of sex. Plasma T4 showed a positive correlation with IG and spawning females, whereas plasma T3 had a similar correlation with 11-KT and the number of early spermiating males in the population. The results demonstrate hormonal signatures of gonadal recrudescence, maturation, and spawning at the population level for promoting recruitment and artificial propagation of hilsa.






Similar content being viewed by others
Data availability
Not applicable.
References
Ahsan DA, Naser MN, Bhaumik U, Hazra S, Bhattacharya SB (2014) Migration, spawning patterns and conservation of hilsa shad (Tenualosa ilisha) in Bangladesh and India. Academic Foundation, New Delhi
Anderson CNK, Hsieh C, Sandin SA, Hewiit R, Hollowed A, Beddington J, May RM, Sugihara G (2008) Why fishing magnifies fluctuations in fish abundance. Nature 452:835–839
Asahina K, Kambegawa A, Higashi T (1995) Development of microtiter plate enzyme-linked immunosorbent assay for 17α, 20β-21-trihydroxy-4-pregnen-l-one, a teleost gonadal steroid. Fish Sci 61:491–494
Baggerman B (1960) Salinity preference, thyroid activity and the seaward migration of four species of Pacific salmon (Oncorhynchus). J Fish Res Board Can 17:295–322
Bhaumik U (2017) Fisheries of Indian Shad (Tenualosa ilisha) in the Hooghly-Bhagirathi stretch of the Ganga River system. Aquat Ecosyst Health Manag 20:130–139
Biran J, Levavi-Sivan B (2018) Endocrine Control of Reproduction, Fish. In: Skinner MK (ed) Encyclopedia of Reproduction, vol 6. Academic Press, Elsevier, pp 362–368
Bizzotto PM, Godinho AL, Vono V, Kynard B, Godinho HP (2009) Influence of seasonal, diel, lunar, and other environmental factors on upstream fish passage in the Igarapava Fish Ladder, Brazil. Ecol Freshw Fish 18:461–472
Bladon A, Myint KH, Ei T, Khine M et al (2019) Spawning seasonality of hilsa (Tenualosa ilisha) in Myanmar’s Ayeyarwady Delta. IIED Working Paper, IIED, London
Bradshaw WE, Holzapfel CM (2007) Evolution of animal photoperiodism. Annu Rev Ecol Evol S 38:1–25
Bromage N, Porter M, Randall C (2001) The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 197:63–98
Brown CL, Doroshov SI, Nunez JM, Hadley C, Vaneenennaam J, Nishioka RS, Bern HA (1988) Maternal triiodothyronine injections cause increases in swim bladder inflation and survival rates in larval striped bass, Morone saxatilis. J Exp Zool 218:168–176
Bucholtz RH, Tomkiewicz J, Dalskov J (2008) Manual to determine gonadal maturity of herring (Clupea harengus L.). In: DTU Aqua-report 197–08. National Institute of Aquatic Resources, Charlottenlund
Carolsfeld J, Scott AP, Collins PM, Sherwood NM (1996) Reproductive steroids during maturation in a primitive teleost, the Pacific herring (Clupea harengus pallasi). Gen Comp Endocrinol 103:331–348
Coward K, Bromage NR (1998) Histological classification of ovarian growth and the dynamics of ovarian recrudescence in Tilapia zillii (Gervais). J Fish Biol 53:285–302
Cyr DG, Eales JG (1996) Interrelationships between thyroidal and reproductive endocrine systems in fish. Rev Fish Biol Fish 6:165–200
Das I, Hazra S, Das S, Giri S, Maity S, Ghosh S (2019) Present status of the sustainable fishing limits for hilsa shad in the northern Bay of Bengal, India. Proc Indian Natl Sci Acad B 89:525–532
De DK (1980) Maturity, fecundity and spawning of post-monsoon run of Hilsa, Hilsa ilisha (Hamilton) in the upper stretches of the Hooghly estuarine system. J Inland Fish Soc India 12:54–63
De Vlaming V, Grossman G, Chapman F (1982) On the use of the gonadosomatic index. Comp Biochem Physiol A 73:31–39
De D, Shyne Anand, Mukherjee S et al (2020) Brood stock development and captive maturation of hilsa (Tenualosa ilisha) in a brackish water pond-based system. J Fish Biol 97:720–733
Funamoto T, Aoki I (2002) Reproductive ecology of Japanese anchovy off the Pacific coast of eastern Honshu, Japan. J Fish Biol 60:154–169
García-López A, Fernández-Pasquier V, Couto E, Canario AVM, Sarasquete C, Martínez-Rodríguez G (2006) Testicular development and plasma sex steroid levels in cultured male Senegalese sole Solea senegalensis Kaup. Gen Comp Endocrinol 147:343–351
Greenblatt M, Brown CL, Lee M, Dauder S, Bern HA (1989) Changes in thyroid hormone levels in eggs and larvae and in iodine uptake by eggs of coho and Chinook salmon, Oncorhynchus kisutch and O. tshawytscha. Fish Physiol Biochem 6(5):262–278
Grier HJ, Uribe-Aranz´abal MC, Patino R (2009) The ovary, folliculogenesis and oogenesis in teleosts. In: Jamieson BGM (ed) Reproductive biology and phylogeny of fishes. Science Publishers, Enfield, pp 25–84
Guerriero G (2007) Seasonal steroids variations and maturity stages in the female Chub Leuciscus cephalaus L. (Pisces, Cyprinidae). Ital J Zool 74:317–324
Hamlin HJ, Milnes MR, Beaulaton CM, Albergotti LC, Guillette LJ (2011) Gonadal stage and sex steroid correlations in Siberian sturgeon, Acipenser baeri, habituated to a semitropical environment. J World Aquacult Soc 42:313–320
Hattori RS, Fernandino JI, Kishii A, Kimura H, Kinno T, Oura M, Somoza GM, Yokota M, Strüssmann CA, Watanabe S (2009) Cortisol-induced masculinization: does thermal stress affect gonadal fate in pejerrey, a teleost fish with temperature-dependent sex determination? PLoS One 4(8):e6548
Hora SL, Nair KK (1940) Further observations on the bionomics and fishery of the Indian shad, Hilsa ilisha (Hamilton), in Bengal waters. Rec Indian Mus 42(1):35–40
Hossain MS, Sharifuzzaman SM, Rouf MA, Pomeroy RS, Hossain MD, Chowdhury SR, AftabUddin S (2019a) Tropical hilsa shad (Tenualosa ilisha): biology, fishery and management. Fish Fish 20(1):44–65
Hossain MAR, Das I, Genevier L, Hazra S, Rahman M, Barange M, Fernandes JA (2019b) Biology and fisheries of Hilsa shad in Bay of Bengal. Sci Total Environ 651:1720–1734
Hunter JR, Goldberg SR (1980) Spawning incidence and batch fecundity in Northern anchovy, Engraulis mordax. U.S. National Marine Fisheries Service Fishery Bulletin 77:641–652
Hunter JR, Macewicz BJ (1985) Measurement of spawning frequency in multiple spawning fishes. NOAA Technical Report NMFS 36:79–94
Jhingran VG, Natarajan AV (1969) A study of the fisheries and fish population of the Chilka Lake during the period 1957-65. J Inland Fish Soc India 1:49–126
Karppinen P, Erkinaro J, NiemelaE, Moen K, Okland F (2004) Return migration of one-sea-winter Atlantic salmon in the River Tana. J Fish Biol 64:1179–1192
Kazeto Y, Tosaka R, Matsubara H, Ijiri S, Adachi S (2011) Ovarian steroidogenesis and the role of sex steroid hormones on ovarian growth and maturation of the Japanese eel. J Steroid Biochem Mol Biol 127:149–154
Koya Y, Soyano K, Yamamoto K, Obana H, Matsubara T (2002) Testicular development and serum profiles of steroid hormone levels in captive male Pacific herring Clupea pallasii during their first maturational cycle. Fish Sci 68:1099–1105
Kuparinen A, O’Hara RB, Merila J (2009) Lunar periodicity and the timing of river entry in Atlantic salmon Salmo salar. J Fish Biol 74:2401–2408
Lam TJ (1994) Hormones and egg/larval quality in fish. J World Aquac Soc 25:2–12
Leder EH, Danzmann RG, Ferguson MM (2006) The candidate gene, clock, localizes to a strong spawning time quantitative trait locus region in rainbow trout. J Hered 97:74–80
Lokman PM, Harris B, Kusakabe M, Kime DE, Schulz RW, Adachi S, Young G (2002) 11-Oxygenated androgens in female teleosts: prevalence, abundance and life history implications. Gen Comp Endocrinol 129:1–12
Lowerre-Barbieri SK, Ganias K, Saborido-Rey F, Murua H, Hunter JR (2011) Reproductive timing inmarine fishes: variability, temporal scales, and methods. Mar Coast Fish 3(1):71–91
Lubzens E, Young G, Bobe J, Cerda J (2010) Oogenesis in teleosts: how fish eggs are formed. Gen Comp Endocrinol 165(3):367–389
Matsubara T, Honda S, Soyano K, Wada T (1992) Seasonal changes in testicular developmental stages and serum levels of steroid hormones in the rearing male Japanese sardine (Sardinops melanostictus). Bull Hokkaido Natl Fish Res Inst 56:7–16
Matsuyama M, Adachi S, Nagahama Y, Kitajima C, Matsuura S (1991) Testicular development and serum levels of gonadal steroids during the annual reproductive cycle of captive Japanese sardine. Jpn J Ichthyol 37:381–390
McBride RS, Ferreri R, Towle EK, Boucher JM, Basilone G (2016) Yolked oocyte dynamics support agreement between determinate- and indeterminate-method estimates of annual fecundity for a north-eastern United States population of American shad. PLoS One 11:e0164203
Miah MS (2015) Climatic and anthropogenic factors changing spawning pattern and production zone of Hilsa fishery in the Bay of Bengal. Weather Clim Extremes 7:109–115
Miah MS, Rahman MA, Haldar GC (1999) Analytical approach to the spawning ground of Hilsa Tenualosa ilisha (Ham.) in Bangladesh water. Indian J Anim Sci 69:141–144
Miura T, Yamauchi K, Takahashi H, Nagahama Y (1991) Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica). Proc Natl Acad Sci USA 88:5774–5778
Morais RDVS, Nóbrega RH, Gómez-Gonzáles NE, Schmidt R, Bogerd J, França LR, Schulz RW (2013) Thyroid hormone stimulates the proliferation of sertoli cells and single type a spermatogonia in adult zebrafish (Danio rerio) testis. Endocrinol 154:4635–4676
Munro AD, Scott AP, Lam TJ (1990) Reproductive seasonality in teleosts: environmental influences. CRC Press, Boca Raton
Murua H, Saborido-Rey F (2003) Female reproductive strategies of marine fish species of the North Atlantic. J Northwest Atl Fish Soc 33:23–31
Mylonas CC, Sullivan CV, Hinshaw JM (1994) Thyroid hormones in brown trout (Salmo trutta) reproduction and early development. Fish Physiol Biochem 13:485–493
Mylonas CC, Scott AP, Zohar Y (1997) Plasma gonadotropin 11, sex steroids, and thyroid hormones in wild striped bass (Morone saxarilis) during spermiation and final oocyte maturation. Gen Comp Endocrinol 108:223–236
Nagahama Y (1994) Endocrine regulation of gametogenesis in fish. Int J Dev Biol 38:217–229
Panhwar SK, Siddiqui G, Zarrien A (2011) Reproductive pattern and some biological features of anadromous fish Tenualosa ilisha from Pakistan. Indian J Geo-Mar Sci 40:687–696
Patiño R, Sullivan CV (2002) Ovarian follicle growth, maturation, and ovulation in teleost fish. Fish Physiol Biochem 26:57–70
Pavlov DA, Emel’yanova NG, Novikov GG (2009) Reproductive dynamics. In: Jakobsen T, Fogarty MJ et al (eds) Fish reproductive biology. Wiley-Blackwell Scientific Publications, Chichester, pp 48–90
Plaza G, Sakaji H, Honda H, Hirota Y, Nashida K (2007) Spawning pattern and type of fecundity in relation to ovarian allometry in the round herring, Etrumeus teres. Mar Biol 152:1051–1064
Pramanick K, Kundu S, Paul S, Mallick B, Roy Moulik S, Pal P, Mukherjee D (2013) Changes in plasma steroid levels during oocyte development in Indian shad, Tenulosa ilisha (Hamilton, 1822): role of gonadotropins on in vitro steroid production and development of oocyte maturational competence. Anim Reprod Sci 141:177–188
Quinn TP, Adams DJ (1996) Environmental changes affecting the migratory timing of American shad and sockeye salmon. Ecology 77:1151–1162
Rahman MJ, Rahman MA, Bhaumik U (2012) Biology and ecology of Hilsa Shad, Tenualosa ilisha (Ham.). Regional Workshop on Hilsa: Potential for Aquaculture, pp. 1–39 (16–17 September 2012. Dhaka, Bangladesh)
Rinchard J, Dabrowski K, Ottobre J (2001) Sex steroids in plasma of lake white fish Coregonus clupeaformis during spawning in Lake Erie. Comp Biochem Physiol C 129:65–74
Sahoo AK, Wahab MW, Phillips M et al (2018) Breeding and culture status of Hilsa (Tenualosa ilisha, Ham. 1822) in South Asia: a review. Rev Aquac 10:96–110
Sajina AM, Suresh VR, Sandhya KM, Mukherjee J, Manna RK, Banik SK, Maity T, Samanta R (2020) Growth overfishing of hilsa shad in Hooghly-Bhagirathi river system, India: Assessment and management implications. Indian J Fish 67:1–7
Scott AP, Sumpter JP, Stacey N (2010) The role of the maturation inducing steroid, 17,20b-dihydroxypregn-4-en-3-one, in male fishes: a review. J Fish Biol 76:183–224
Schulz RW, de França LR, Lareyre JJ, LeGac, F, Chiarini-Garcia H, Nobrega RH, Miura T (2010) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411
Smith B, Walker KF (2004) Spawning dynamic of common carp in the River Murray, South Australia, shown by macroscopic and histological staging of gonads. J Fish Biol 64:336–354
Shifat R, Begum A, Khan H (2003) Use of RAPD fingerprinting for discriminating two populations of Hilsa shad (Tenualosa ilisha Ham.) from inland Rivers of Bangladesh. J Biochem Mol Biol 36:462–467
Tahara D, Hatano R, Iwatani H, Koya Y, Hayakawa Y (2010) Annual changes in testicular development and occurrence of parasperm in the male reproductive organ of four spine sculpin, Cottus kazika. Ichthyol Res 57:62–70
Tomkiewicz J, Kofoed TMN, Pederson JS (2011) Assessment of testis development during induced spermatogenesis in European eel Anguilla anguilla. Marine and Coastal Fisheries: Dynamics, Management, and Ecosystem Science [online serial] 3:106–118
Tyler CR, Sumpter JP (1996) Oocyte growth and development in teleosts. Rev Fish Biol Fisheries 6:287–318
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte growth in teleosts. Am Zool 21(2):325–343
Wang W, Zhu H, Tian Z, Sun A, Dong Y, Dong T, Hu H (2020) Effects of 11-ketotestosterone on development of the previtellogenic ovary in the Sterlet, Acipenser ruthenus. Front Endocrinol 11:115
Whitehead P (1985) Clupeoid fishes of the world (suborder Clupeioidei).An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf herrings. FAO Fisheries Synopsis, 7(125)
Workman RD, Hayes DB, Coon TG (2002) A model of steelhead movement in relation to water temperature in two Lake Michigan tributaries. Trans Am Fish Soc 131:463–475
Wright PJ (2007) Understanding the maturation process for field investigations of fisheries-induced evolution. Mar Ecol Prog Ser 335:279–283
Yamahira K (2001) Experimental examination of interpopulation variation in reproductive timing of a fish. Oecologia 128:389–399
Zhang J, Takita T, Zhang C (2009) Reproductive biology of Ilisha elongate (Teleostei: Pristigasteridae) in Ariake Sound, Japan: implications for estuarine fish conservation in Asia. Estuar Coast Shelf Sci 81:105–113
Acknowledgements
The authors are grateful to Dr. Gopal Krishna, Director and Vice chancellor, ICAR-Central Institute of Fisheries Education, Mumbai for providing necessary facilities for carrying out the research. This research was funded by National Agriculture Science Fund, Indian Council of Agricultural Research, New Delhi under the project no.WQ-3021.
Funding
This research was funded by National Agriculture Science Fund, Indian Council of Agricultural Research, New Delhi under the grant no. NFBSFARA/WQ-3021.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: S.D.G.; Performed the experiments: S.K.R, S.D.G., S.D., and G.H.P.; Analyzed the data: S.K.R., S.D., S.D.G., G.H.P., and V.R.S. Wrote the paper: S.D.G. and S.K.R. Review and editing: S.D.G., S.D. and S.K.R.
Corresponding author
Ethics declarations
Ethics approval
The study was undertaken with the approval of statutory authorities of the ICAR-Central Institute of Fisheries Education, Mumbai, India (University under Sect. 3 of University Grants Commission Act, and ISO 9001:2008 certified).
Consent for publication
All authors approved the final manuscript for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ray, S.K., Dutta, S., Pailan, G.H. et al. Hormonal signatures of gonad maturity and seasonality of spawning in migrating hilsa, Tenualosa ilisha. Environ Biol Fish 105, 37–53 (2022). https://doi.org/10.1007/s10641-021-01186-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-021-01186-5