Abstract
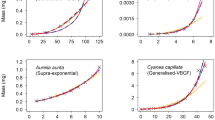
Growth in fishes is usually modelled by a function encapsulating a common growth mechanism across ages. However, several theoretical works suggest growth may comprise two distinct mechanistic phases arising from changes in reproductive investment, diet, or habitat. These models are termed two-state or biphasic, where acceleration in growth typically changes around some transition age. Such biphasic models have already been successfully applied in elasmobranch species, where such transitions are detectable from length-at-age data alone, but where estimation has assumed normally distributed errors, which is inappropriate for such slow-growing and long-lived fishes. Using recent advances in growth parameter estimation, we implement a biphasic growth model with asymmetric and heavy-tailed errors. We use data from six datasets, encompassing four species of elasmobranchs, to compare the performance of the von Bertalanffy and biphasic models under normal, skew-normal, and Student-t error distributions. Conditional expectation maximization estimation proves both effective and efficient in this context. Most datasets analysed here supported asymmetric and heavy-tailed errors and biphasic growth, producing parameter estimates different from previous studies.



Similar content being viewed by others
References
Abid SH, Quaez UJ, Contreras-Reyes JE (2021) An information-theoretic approach for multivariate skew-t distributions and applications. Mathematics 9:146
Araya M, Cubillos LA (2006) Evidence of two-phase growth in elasmobranchs. Environ Biol Fish 77:293–300
Boukal DS, Dieckmann U, Enberg K, Heino M, Jørgensen C (2014) Life-history implications of the allometric scaling of growth. J Theor Biol 359:199–207
Charnov EL (1993) Life history invariants. Oxford University Press, London
Contreras-Reyes JE, Arellano-Valle RB, Canales TM (2014) Comparing growth curves with asymmetric heavy-tailed errors: Application to the southern blue whiting (Micromesistius australis). Fish Res 159:88–94
Contreras-Reyes JE, Quintero FOL, Wiff R (2018a) Bayesian modeling of individual growth variability using back-calculation: application to pink cusk-eel (Genypterus blacodes) off Chile. Ecol Model 385:145–153
Contreras-Reyes JE, Quintero FOL, Yáñez AA (2018b) Towards age determination of Southern King Crab (Lithodes santolla) off Southern Chile using flexible mixture modeling. J Mar Sci Eng 6:157
Cortés E (2000) Life history patterns and correlations in sharks. Rev Fish Sci 8:299–344
Czarnoleski M, Kozlowski J (1998) Do Bertalanffy’s growth curves result from optimal resource allocation?. Ecol Lett 1:5–7
Frisk MG, Miller TJ, Fogarty MJ (2001) Estimation and analysis of biological parameters in elasmobranch fishes: a comparative life history study. Can J Fish Aquat Sci 58:969–981
Huusko A, Mäki-Petäys A, Stickler M, Mykrä H (2011) Fish can shrink under harsh living conditions. Funct Ecol 25:628–633
Jensen CF, Natanson LJ, Pratt Jr HL, Kohler N, Campana SE (2002) The reproductive biology of the porbeage shark (Lamna nasus) in the western North Atlantic Ocean. Fish Bull 100:727–738
Kimura DK (1990) Testing nonlinear regression parameters under heteroscedastic, normally distributed errors. Biometrics 46:697–708
Kusher DI, Smith SE, Cailliet GM (1992) Validated age and growth of the leopard shark, Triakis semifasciata, with comments on reproduction. Environ Biol Fish 35:187– 203
Lester NP, Shuter BJ, Abrams PA (2004) Interpreting the von Bertalanffy model of somatic growth in fishes: the cost of reproduction. Proc Roy Soc Lond B 271:1625– 1631
Liu C, Rubin DB (1994) The ECME algorithm: a simple extension of EM and ECM with faster monotone convergence. Biometrika 81:633–648
Minte-Vera CV, Maunder MN, Casselman JM, Campana SE (2016) Growth functions that incorporate the cost of reproduction. Fish Res 180:31–44
Montenegro C, Branco M (2016) Bayesian state-space approach to biomass dynamic models with skewed and heavy-tailed error distributions. Fish Res 181:48–62
Morales-Nin B (2000) Review of the growth regulation processes of otolith daily increment formation. Fish Res 46:53–67
Natanson LJ, Mello JJ, Campana SE (2002) Validated age and growth of the porbeagle shark (Lamna nasus) in the western North Atlantic Ocean. Fish Bull 100:266–278
Ohnishi S, Yamakawa T, Okamura H, Akamine T (2012) A note on the von Bertalanffy growth function concerning the allocation of surplus energy to reproduction. Fish Bull 110:223–229
Paloheimo JE, Dickie LM (1965) Food and growth of fishes I. A growth curve derived from experimental data. J Fish Res Board Can 22:521–542
Quince C, Abrams PA, Shuter BJ, Lester NP (2008) Biphasic growth in fish I: theoretical foundations. J Theor Biol 254:197–206
Quintero FOL, Contreras-Reyes JE, Wiff R, Arellano-Valle RB (2017a) Flexible Bayesian analysis of the von Bertalanffy growth function using log-skew-t distribution. Fish Bull 115:13–26
Quintero FOL, Contreras-Reyes JE, Wiff R (2017b) Incorporating uncertainty into a length-based estimator of natural mortality in fish populations. Fish Bull 115:355–364
R Core Team (2019) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Siegfried KI, Sansó B (2006) Two Bayesian methods for estimating parameters of the von Bertalanffy growth equation. Environ Biol Fish 77:301–308
Skomal GB, Natanson LJ (2003) Age and growth of the blue shark (Prionace glauca) in the North Atlantic Ocean. Fish Bull 101:627–639
Soriano M, Moreau J, Hoenig JM, Pauly D (1992) New functions for the analysis of two-phase growth of juvenile and adult fishes, with application to Nile perch. Trans Amer Fish Soc 121:486–493
Saure L, Hyvärinen H (1965) Seasonal changes in the histological structure of the spinal column of Sorex Araneus (L.) Nature 208:705–706
Sulikowski JA, Morin MD, Suk SH, Howell WH (2003) Age and growth estimates of the winter skate (Leucoraja ocellata) in the western Gulf of Maine. Fish Bull 101:405–413
Taylor NG, Walters CJ, Martell SJD (2005) A new likelihood for simultaneously estimating von Bertalanffy growth parameters, gear selectivity and natural and fishing mortality. Can J Fish Aquat Sci 62:215–223
von Bertalanffy L (1938) A quantitative theory of organic growth (Inquiries on growth laws. II). Hum Biol 10:181–213
Walker TI, Taylor BL, Hudson RJ, Cottier JP (1998) The phenomenon of apparent change of growth rate in gummy shark (Mustelus antarcticus) harvested off southern Australia. Fish Res 39:139–163
Wiff R, Roa-Ureta R (2008) Predicting the slope of the allometric scaling of consumption rates in fish using the physiology of growth. Mar Fresh Res 58:912–921
Wilson KL, Honsey AE, Moe B, Venturelli P (2018) Growing the biphasic framework: Techniques and recommendations for fitting emerging growth models. Meth Ecol Evol 9:822–833
Wikelski M, Thom C (2000) Marine iguanas shrink to survive El Niño. Nature 403:37–38
Wourms JP, Demski LS (1993) The reproduction and development of sharks, skates, rays and ratfishes: introduction, history, overview, and future prospects. Environ Biol Fish 38:7–21
Acknowledgements
The authors thank the advisory editor and two anonymous referees for their helpful comments and suggestions which greatly improved and early version of this manuscript.
Funding
J.E. Contreras-Reyes’ research was fully supported by FONDECYT (Chile) grant No. 11190116. R. Wiff was funded by CAPES Project Conicyt FB 0002 (2014) and by ANID - Programa Iniciativa Científica Milenio - Código ICN2019_015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The authors have adhered to general guidelines for ethical use of animals in research and to the legal requirements and any other institutional guidelines promulgated in Chile. In addition, we would like to thank the authors who facilitated their information (Section 2.1) to us to conduct the analyses.
Conflict of interest
The authors declare no competing interests.
Additional information
Data availability
Data used in this paper will be made available upon reasonable request from the corresponding author.
Code availability
All R codes used in this paper are available upon request from the corresponding author.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Contreras-Reyes, J.E., Wiff, R., Soto, J. et al. Biphasic growth modelling in elasmobranchs based on asymmetric and heavy-tailed errors. Environ Biol Fish 104, 615–628 (2021). https://doi.org/10.1007/s10641-021-01100-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-021-01100-z