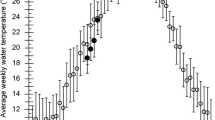
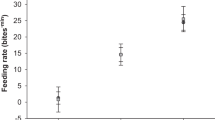
Abstract
Temperature is a key determinant that governs fish survival, reproduction, growth and metabolism. In freshwater ecosystems, anthropogenic influences have resulted in acute and prolonged temperature changes which lead to lethal and sub-lethal impacts on the biota that occupy these environments. We assessed the effects of temperature on somatic and otolith growth and development of three species of native Australian freshwater fish (silver perch Bidyanus bidyanus, trout cod Maccullochella macquariensis and golden perch Macquaria ambigua) to simulate how thermal pollution from the release of unseasonably cold water from thermally stratified dams in Australian freshwater ecosystems may impact fish at critical life-history stages. Fish (31 days post-hatch) were exposed to four temperature treatments (13, 16, 20, 24 °C) for 30 days. Low temperatures resulted in reduced somatic growth, with no growth observed in silver perch and golden perch held at 13 °C over 30 days. Somatic growth was highest at the upper temperature of 24 °C. Morphological assessment of fish size reiterated that low water temperatures resulted in reduced body size, particularly in terms of body width and head size. Low temperatures were associated with reduced otolith growth in all species, however a somatic-otolith size relationship was maintained for all species in measures of otolith weight, area, length and perimeter. The sub-lethal impacts observed in our study are likely to manifest at the population level through a reduced capacity of larvae and juveniles to avoid size-dependent predation, a narrower range of prey sources due to extended gape-limited feeding and, ultimately, poorer survival and recruitment.








Similar content being viewed by others
References
Abbink W, Garcia AB, Roques JA, Partridge GJ, Kloet K, Schneider O (2012) The effect of temperature and pH on the growth and physiological response of juvenile yellowtail kingfish Seriola lalandi in recirculating aquaculture systems. Aquaculture 330:130–135
Astles KL, Winstanley RK, Harris JH, Gehrke PC (2003) Experimental study of the effects of cold water pollution on native fish. NSW Fisheries Research Institute, Sydney, p 54
Barber M, Jenkins G (2001) Differential effects of food and temperature lead to decoupling of short-term otolith and somatic growth rates in juvenile King George whiting. J Fish Biol 58(5):1320–1330
Baumann H, Peck MA, Herrmann J-P (2005) Short-term decoupling of otolith and somatic growth induced by food level changes in postlarval Baltic sprat, Sprattus sprattus. Mar Freshw Res 56(5):539–547
Beitinger TL, Bennett WA, McCauley RW (2000) Temperature tolerances of north American freshwater fishes exposed to dynamic changes in temperature. Environ Biol Fish 58(3):237–275
Bermejo S, Monegal B, Cabestany J (2007) Fish age categorization from otolith images using multi-class support vector machines. Fish Res 84(2):247–253
Brett JR (1971) Energetic responses of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerkd). Am Zool 11(1):99–113
Brown A, Busby M, Mier K (2001) Walleye Pollock Theragra chalcogramma during transformation from the larval to juvenile stage: otolith and osteological development. Mar Biol 139(5):845–851
Buckmeier DL, Irwin ER, Betsill RK, Prentice JA (2002) Validity of otoliths and pectoral spines for estimating ages of channel catfish. N Am J Fish Manag 22(3):934–942
Buentello JA, Gatlin DM III, Neill WH (2000) Effects of water temperature and dissolved oxygen on daily feed consumption, feed utilization and growth of channel catfish (Ictalurus punctatus). Aquaculture 182(3–4):339–352
Burton C (2000) Assessment of the water temperature regime of the Macquarie River, central west, New South Wales. NSW Department of Land and Water Conservation, Dubbo
Campana SE (1999) Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser 188:263–297
Campana SE, Neilson JD (1985) Microstructure of fish otoliths. Can J Fish Aquat Sci 42(5):1014–1032
Childs MR, Clarkson RW (1996) Temperature effects on swimming performance of larval and juvenile Colorado squawfish: implications for survival and species recovery. Trans Am Fish Soc 125(6):940–947
Clarkson RW, Childs MR (2000) Temperature effects of hypolimnial-release dams on early life stages of Colorado River basin big-river fishes. Copeia 2:402–412
Comte L, Olden JD (2017) Evolutionary and environmental determinants of freshwater fish thermal tolerance and plasticity. Glob Chang Biol 23(2):728–736
Cordes JF, Allen LG (1997) Estimates of age, growth, and settlement from otoliths of young-of-the-year kelp bass (Paralabrax clathratus). Bull South Calif Acad Sci 96:43–60
Domenici P (2001) The scaling of locomotor performance in predator-prey encounters: from fish to killer whales. Comp Biochem Physiol A Mol Integr Physiol 131(1):169–182
Domenici P, Blake R (1997) The kinematics and performance of fish fast-start swimming. J Exp Biol 200(8):1165–1178
Fablet R, Le Josse N (2005) Automated fish age estimation from otolith images using statistical learning. Fish Res 72(2–3):279–290
Fausch KD, White RJ (1986) Competition among juveniles of coho salmon, brook trout, and brown trout in a laboratory stream, and implications for Great Lakes tributaries. Trans Am Fish Soc 115(3):363–381
Fernández-Montero A, Caballero MJ, Torrecillas S, Tuset VM, Lombarte A, Ginés RR, Izquierdo M, Robaina L, Montero D (2018) Effect of temperature on growth performance of greater amberjack (Seriola dumerili Risso 1810) juveniles. Aquac Res 49(2):908–918
Ferrari R (1987) Colorado River water temperature modeling below Glen Canyon Dam. Glen Canyon Environmental Studies Report GCES/13/87. Durango Projects Office, Upper Colorado Region, US Bureau of Reclamation
Fey D (2006) The effect of temperature and somatic growth on otolith growth: the discrepancy between two clupeid species from a similar environment. J Fish Biol 69(3):794–806
Fisher R, Leis JM, Clark DL, Wilson SK (2005) Critical swimming speeds of late-stage coral reef fish larvae: variation within species, among species and between locations. Mar Biol 147(5):1201–1212
Folkvord A, Johannessen A, Moksness E (2004) Temperature-dependent otolith growth in Norwegian spring-spawning herring (Clupea harengus L.) larvae. Sarsia N Atl Mar Sci 89(5):297–310
Francis MP, Williams MW, Pryce AC, Scott SG (1993) Uncoupling of otolith and somatic growth in Pagrus auratus (Sparidae). Fish Bull 91(1):159
Gagliano M, McCormick MI (2004) Feeding history influences otolith shape in tropical fish. Mar Ecol Prog Ser 278:291–296
Gauldie R, Nelson D (1990) Otolith growth in fishes. Comp Biochem Physiol A Physiol 97(2):119–135
Gehrke PC (1988) Feeding energetics and angling catches of spangled perch, Leiopotherapon unicolor (Günther 1859),(Percoidei: Teraponidae). Mar Freshw Res 39(4):569–577
Gray R, Jones HA, Hitchcock JN, Hardwick L, Pepper D, Lugg A, Seymour JR, Mitrovic SM (2019) Mitigation of cold-water thermal pollution downstream of a large dam with the use of a novel thermal curtain. River Res Appl 35:855–866
Green BS, Fisher R (2004) Temperature influences swimming speed, growth and larval duration in coral reef fish larvae. J Exp Mar Biol Ecol 299(1):115–132
Gunderson AR, Stillman JH (2015) Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc R Soc B Biol Sci 282(1808):20150401
Hanna RB, Saito L, Bartholow JM, Sandelin J (1999) Results of simulated temperature control device operations on in-reservoir and discharge water temperatures using CE-QUAL-W2. Lake Reserv Manag 15(2):87–102
Hoff GR, Fuiman LA (1993) Morphometry and composition of red drum otoliths: changes associated with temperature, somatic growth rate, and age. Comp Biochem Physiol A Physiol 106(2):209–219
Imsland A, Sunde L, Folkvord A, Stefansson S (1996) The interaction of temperature and fish size on growth of juvenile turbot. J Fish Biol 49(5):926–940
Jobling M (1983) Growth studies with fish-overcoming the problems of size variation. J Fish Biol 22(2):153–157
Jobling M (1995) Fish bioenergetics. Oceanogr Lit Rev 9(42):785
Jobling M (ed) (1997) Temperature and growth: modulation of growth rate via temperature change. Cambridge University Press, Cambridge
Johnson TB, Evans DO (1990) Size-dependent winter mortality of young-of-the-year white perch: climate warming and invasion of the Laurentian Great Lakes. Trans Am Fish Soc 119(2):301–313
Johnson TB, Evans DO (1996) Temperature constraints on overwinter survival of age-0 white perch. Trans Am Fish Soc 125(3):466–471
Jonassen T, Imsland A, Stefansson S (1999) The interaction of temperature and fish size on growth of juvenile halibut. J Fish Biol 54(3):556–572
Jones CM (1992) Development and application of the otolith increment technique Otolith microstructure examination and analysis. Canad Spec Publ Fish Aquat Sci 117:1–11
Karlou-Riga C (2000) Otolith morphology and age and growth of Trachurus mediterraneus (Steindachner) in the eastern Mediterranean. Fish Res 46(1–3):69–82
Kitchell JF, Stewart DJ, Weininger D (1977) Applications of a bioenergetics model to yellow perch (Perca flavescens) and walleye (Stizostedion vitreum vitreum). J Fish Board Can 34(10):1922–1935
Koehn J (2001) Ecological impacts of cold water releases on fish and ecosystem processes. In: Thermal Pollution of the Murray-Darling Waterways: Workshop Held at Lake Hume:18–19. (ed. B. Phillips). Inland Rivers Networkand World Wide Fund for Nature Australia, Sydney
Libungan L, Pálsson S (2015a) ShapeR: collection and analysis of Otolith shape data, R package version 0.1-5. Available at http://github.com/lisalibungan/shapeR
Libungan LA, Pálsson S (2015b) ShapeR: an R package to study otolith shape variation among fish populations. PLoS One 10(3):e0121102
Lugg A, Copeland C (2014) Review of cold water pollution in the Murray-Darling basin and the impacts on fish communities. Ecol Manag Restor 15(1):71–79. https://doi.org/10.1111/emr.12074
Lyon J, Ryan T, Scroggie M (2008) Effects of temperature on the fast-start swimming performance of an Australian freshwater fish. Ecol Freshw Fish 17(1):184–188
Michie LE, Hitchcock JN, Thiem JD, Boys CA, Mitrovic SM (2020) The effect of varied dam release mechanisms and storage volume on downstream river thermal regimes. Limnologica 81:125760
Modin J, Fagerholm B, Gunnarsson B, Pihl L (1996) Changes in otolith microstructure at metamorphosis of plaice, Pleuronectes platessa L. ICES J Mar Sci 53(4):745–748
Morita K, Fukuwaka M, Tanimata N, Yamamura O (2010) Size-dependent thermal preferences in a pelagic fish. Oikos 119(8):1265–1272
Morrison CM, Kunegel-Lion M, Gallagher CP, Wastle RJ, Lea EV, Loewen TN, Reist JD, Howland KL, Tierney KB (2019) Decoupling of otolith and somatic growth during anadromous migration in a northern salmonid. Can J Fish Aquat Sci 76(11):1940–1953
Morrongiello JR, Thresher RE, Smith DC (2012) Aquatic biochronologies and climate change. Nat Clim Chang 2(12):849–857
Mosegaard H, Svedäng H, Taberman K (1988) Uncoupling of somatic and otolith growth rates in Arctic char (Salvelinus alpinus) as an effect of differences in temperature response. Can J Fish Aquat Sci 45(9):1514–1524
Myrick CA, Cech JJ (2000) Swimming performances of four California stream fishes: temperature effects. Environ Biol Fish 58(3):289–295
Neuman M, Witting D, Able K (2001) Relationships between otolith microstructure, otolith growth, somatic growth and ontogenetic transitions in two cohorts of windowpane. J Fish Biol 58(4):967–984
Nishimura A, Yamada J (1984) Age and growth of larval and juvenile walleye Pollock, Theragra chalcogramma (Pallas), as determined by otolith daily growth increments. J Exp Mar Biol Ecol 82(2–3):191–205
NSW-CWPIG (2012) Cold water pollution strategy in NSW - report on the implementation of stage one. Published by NSW Department of Primary Industries, Water
Ojanguren A, Brana F (2003) Effects of size and morphology on swimming performance in juvenile brown trout (Salmo trutta L.). Ecol Freshw Fish 12(4):241–246
Oksanen J et al (2016) Vegan: community ecology package. R package version 2. 4-3. Available at http://CRAN.R-project.org/package=vegan
Pannella G (1971) Fish otoliths: daily growth layers and periodical patterns. Science 173(4002):1124–1127
Pérez-Casanova JC, Lall SP, Gamperl AK (2009) Effect of feed composition and temperature on food consumption, growth and gastric evacuation of juvenile Atlantic cod (Gadus morhua L.) and haddock (Melanogrammus aeglefinus L.). Aquaculture 294(3–4):228–235
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Radtke RL (1989) Larval fish age, growth, and body shrinkage: information available from otoliths. Can J Fish Aquat Sci 46(11):1884–1894
Rice JA, Crowder LB, Holey ME (1987) Exploration of the mechanisms regulating larval survival in Lake-Michigan bloater; a recruitment analysis based on characteristics of individual larvae. Trans Am Fish Soc 116(5):703–718. https://doi.org/10.1577/1548-8659(1987)116<703:eomrls>2.0.co;2
Roessig JM, Woodley CM, Cech JJ, Hansen LJ (2004) Effects of global climate change on marine and estuarine fishes and fisheries. Rev Fish Biol Fish 14(2):251–275
Ryan T, Lennie, R, Lyon J, O'Brien T (2003) Thermal rehabilitation of the southern Murray-Darling basin. Final Report to Agriculture, Forestry, Fisheries Australia
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7):676–682
Secor DH, Dean JM, Baldevarona RB (1989) Comparison of otolith growth and somatic growth in larval and juvenile fishes based on otolith length/fish length relationships. Rapp Pv Réun Cons Int Explor Mer 191:431–438
Sherman B (2000) Scoping options for mitigating cold water discharges from dams. CSIRO Land and Water, Canberra
Sherman B, Todd CR, Koehn JD, Ryan T (2007) Modelling the impact and potential mitigation of cold water pollution on Murray cod populations downstream of Hume dam, Australia. River Res Appl 23(4):377–389. https://doi.org/10.1002/rra.994
Sogard SM (1997) Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull Mar Sci 60(3):1129–1157
Sponaugle S (2010) Otolith microstructure reveals ecological and oceanographic processes important to ecosystem-based management. Environ Biol Fish 89(3–4):221–238
Starrs D, Ebner B, Lintermans M, Fulton C (2011) Using sprint swimming performance to predict upstream passage of the endangered Macquarie perch in a highly regulated river. Fish Manag Ecol 18(5):360–374
Starrs D, Ebner BC, Fulton C (2013) Can backcalculation models unravel complex larval growth histories in a tropical freshwater fish? J Fish Biol 83(1):96–110
Todd CR, Ryan T, Nicol SJ, Bearlin AR (2005) The impact of cold water releases on the critical period of post-spawning survival and its implications for Murray cod (Maccullochella peelii peelii): a case study of the Mitta Mitta River, southeastern Australia. River Res Appl 21(9):1035–1052. https://doi.org/10.1002/rra.873
Tonkin Z, King AJ, Ramsey D (2008a) Otolith increment width responses of juvenile Australian smelt Retropinna semoni to sudden changes in food levels: the importance of feeding history. J Fish Biol 73(4):853–860
Tonkin Z, King AJ, Robertson A (2008b) Validation of daily increment formation and the effects of different temperatures and feeding regimes on short-term otolith growth in Australian smelt Retropinna semoni. Ecol Freshw Fish 17(2):312–317
USDI (1999) Glen canyon dam modifications to control downstream temperatures: plan and draft environmental assessment. USDI, Bureau of Reclamation, Upper Colorado Region, Salt Lake City
Van Vliet MT et al (2013) Global river discharge and water temperature under climate change. Glob Environ Chang 23(2):450–464
Verones F, Hanafiah MM, Pfister S, Huijbregts MA, Pelletier GJ, Koehler A (2010) Characterization factors for thermal pollution in freshwater aquatic environments. Environ Sci Technol 44(24):9364–9369
Videler J, Wardle C (1991) Fish swimming stride by stride: speed limits and endurance. Rev Fish Biol Fish 1(1):23–40
Ward DL, Eugene Maughan O, Bonar SA, Matter WJ (2002) Effects of temperature, fish length, and exercise on swimming performance of age-0 flannelmouth sucker. Trans Am Fish Soc 131(3):492–497
Weber M, Rinke K, Hipsey MR, Boehrer B (2017) Optimizing withdrawal from drinking water reservoirs to reduce downstream temperature pollution and reservoir hypoxia. J Environ Manag 197:96–105. https://doi.org/10.1016/j.jenvman.2017.03.020
Wolter C, Arlinghaus R (2003) Navigation impacts on freshwater fish assemblages: the ecological relevance of swimming performance. Rev Fish Biol Fish 13(1):63–89
Wright P, Metcalfe N, Thorpe J (1990) Otolith and somatic growth rates in Atlantic salmon parr, Salmo salar L: evidence against coupling. J Fish Biol 36(2):241–249
Acknowledgements
The authors would like to pass on their sincere gratitude for the guidance and advice provided by all staff of the Narrandera Fisheries Centre and their generosity in providing use of facilities and resources, in particular Lachie Jess and Matt McLellan, as well as Daniel Wright and Osmar Luiz for assistance with statistical analysis. Financial support for this research was provided by DPI Water and DPI Fisheries, The Fisheries Scientific Committee, The Society for Freshwater Science and the Australian Wildlife Society. Ethics approval for this research was granted by the University of Technology Sydney Animal Care and Ethics Committee under permit ETH18-2580.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Michie, L.E., Thiem, J.D., Facey, J.A. et al. Effects of suboptimal temperatures on larval and juvenile development and otolith morphology in three freshwater fishes: implications for cold water pollution in rivers. Environ Biol Fish 103, 1527–1540 (2020). https://doi.org/10.1007/s10641-020-01041-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-020-01041-z