Abstract
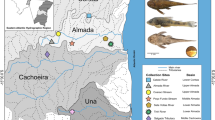
Austrolebias wolterstorffi is a critically endangered annual fish, occurring in temporary ponds in a restricted area of Southern Brazil and Uruguay. Here, we evaluate the levels of genetic diversity and morphometric differentiation presented by A. wolterstorffi, attempting to reconstruct the spatiotemporal scenario by which this species reached their current distribution. Part of the mitochondrial cytochrome b and nuclear rhodopsin genes were characterized and analysed for a set of 122 and 110 specimens, respectively, collected along the entire distribution range of the species. Additionally, shape variations were evaluated for 92 individuals (43 males and 49 females) through geometric morphometric methods. Our analyses demonstrated several cases of significantly high levels of genetic differentiation among individual populations, in an isolation-by-distance pattern of divergence, with at least six different population groups along the Patos-Mirim lagoon. These groups differed by a minimum of 0.9% and a maximum of 2.6% of corrected cyt b nucleotide distances and did not share any mitochondrial haplotype. Such a pattern, added to the slight morphometric differentiation detected for most of the groups, suggests the occurrence of incipient speciation as consequence of allopatric fragmentation. The chronophylogenetic tree performed with the concatenated dataset supported independent oriental and occidental colonization routes, with the population located in the northwest part of the Rio Grande do Sul coastal plain presenting the most ancient divergence. In general, the recovered biogeographic patterns are highly consistent with the records of Quaternary climatic changes and depositional events that have occurred along the area inhabited by the studied species. This allowed us to establish a molecular clock calibration system for Neotropical annual fish. Thus, although the taxonomic status of each of the detected population units needs further study, it is clear that independent conservation strategies must be taken in each of the major areas covered by this study, most of which are located in Brazil.




Similar content being viewed by others
References
Adams DC, Otarola-Castillo E (2013) Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol 4:393–399
Avise JC (1994) Molecular markers, natural history and evolution. Chapman and Hall, New York, 511 p
Avise JC (2004) Molecular markers, natural history and evolution. 2nd edn. Sinauer Associates, Sunderland
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Bartáková V, Reichard M, Janko K et al (2013) Strong population genetic structuring in an annual fish, Nothobranchius furzeri, suggests multiple savannah refugia in southern Mozambique. BMC Evol Biol 13:196
Bossi J, Navarro R (1988) Geología del Uruguay. Universidad de la República, Montevideo, 966 p
Brown WM, Jr George M, Wilson AC (1979) Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A 76:1967–1971
Chen WJ, Bonillo C, Lecointre G (2003) Repeatability of clades as a criterion of reliability: a case study for molecular phylogeny of Acanthomorpha (Teleostei) with larger number of taxa. Mol Phylogenet Evol 26:262–288
Corander J, Sirén J, Arjas E (2008) Bayesian spatial modeling of genetic population structure. Comput Stat 23:111–129
Costa WJEM (2006) The South American annual killifish genus Austrolebias (Teleostei: Cyprinodontiformes: Rivulidae): phylogenetic relationships, descriptive, morphology and taxonomic revision. Zootaxa 1213:1–162
Costa WJEM (2008) Catalog of Aplocheiloid killifishes of the world. UFRJ, Rio de Janeiro
Costa WJEM (2010) Historical biogeography of Cynolebiasine annual killifishes inferred from dispersal-vicariance analysis. J Biogeogr 37:1995–2004
Costa WJEM (2013) Historical biogeography of aplocheiloid killifishes (Teleostei: Cyprinodontiformes). Vertebr Zool 63:139–154
Costa WJEM, Amorim PF (2011) A new annual killifish species of the Hypsolebias flavicaudatus complex from the São Francisco River basin, Brazilian Caatinga (Cyprinodontiformes: Rivulidae). Vertebr Zool 61:99–104
Costa WJEM, Amorim PF, Aranha GM (2014) Species limits and DNA barcodes in Nematolebias, a genus of seasonal killifishes threatened with extinction from the Atlantic Forest of South-Eastern Brazil, with description of a new species (Teleostei: Rivulidae). Ichthyol Explor Fres 24:225–236
Costa WJEM, Amorim PF, Mattos JLO (2016) A new species of inseminating seasonal killifish of the Cynopoecilus melanotaenia complex from southern Brazil (Cyprinodontiformes: Rivulidae). Biodivers Data J 4:e6888–17
Costa WJEM, Cheffe MM, Amorim PF (2017) Two new seasonal killifishes of the Austrolebias adloffi group from the Lagoa dos Patos basin, southern Brazil (Cyprinodontiformes: Aplocheilidae). Vertebr Zool 67:139–149
de Sá RO, Berois N, García G (2015) Overview, future challenges, and evolution of Annualism. In: Berois N, García G, de Sá RO (eds) Annual fishes: life history strategy, diversity, and evolution, 1st edn. Press Taylor & Francis group, Boca Raton, pp 309–318
Dupanloup I, Schneider S, Excoffier L (2002) A simulated annealing approach to define the genetic structure of populations. Mol Ecol 11:2571–2581
Drummond AJ, Rambaut A (2007) BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol 7:214
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and windows. Mol Ecol Resour 10:564–567
Frankham R, Ballou JD, Briscoe DA (2004) A primer of conservation genetics. Cambridge University Press, Cambridge
Frankham R, Brook BW, Bradshaw CJA, Traill LW, Spielman D (2013) 50/500 rule and minimum viable populations: response to Jamieson and Allendorf. Trends Ecol Evol 28:187–188
Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147:915–925
García G, Wlasiuk G, Lessa E (2000) High levels of mitochondrial cytochrome b divergence in annual killifishes of the genus Cynolebias (Cyprinodontiformes, Rivulidae). Zool J Linn Soc-Lond 129:93–110
García G, Loureiro M, Berois N et al (2009) Pattern of differentiation in the annual killifish genus Austrolebias (Cyprinodontiformes; Rivulidae) from a biosphere reserve site in South America: a multidisciplinary approach. Biol J Linn Soc 98:620–635
García G, Gutiérrez V, Vergara J, Calviño P, Duarte A, Loureiro M (2012) Patterns of population differentiation in annual killifishes from the Paraná-Uruguay-La Plata basin: the role of vicariance and dispersal. J Biogeogr 39:1707–1719
García G, Gutiérrez V, Ríos N, Turner B, Santiñaque F, López-Carro B, Folle G (2014) Burst speciation processes and genomic expansion in the neotropical annual killifish genus Austrolebias (Cyprinodontiformes, Rivulidae). Genetica 142:87–98
García G, Gutiérrez V, Ríos N, de Sá RO (2015) Comparative Phylogeographic patterns in Austrolebias from different south American basins. In: Berois N, García G, de Sá RO (eds) Annual fishes: life history strategy, diversity, and evolution, 1st edn. Press Taylor & Francis group, Boca Raton, pp 259–279
ICMBio, Instituto Chico Mendes de Conservação da Biodiversidade (2013) Sumário executivo do plano de ação nacional para a conservação dos peixes Rivulídeos ameaçados de extinção. Brasília. http://www.icmbio.gov.br/portal/biodiversidade/fauna-brasileira/plano-de-acao/2833-plano-de-acao-nacional-para-a-conservacao-dos-rivulideos.html. Accessed 16 November 2016
Jowers MJ, Cohen BL, Downie JR (2008) The cyprinodont fish Rivulus (Aplocheiloidei: Rivulidae) in Trinidad and Tobago: molecular evidence for marine dispersal, genetic isolation and local differentiation. J Zool Syst Evol Res 46:48–55
Kuhner MK (2006) LAMARC 2.0: maximum likelihood and Bayesian estimation of population parameters. Bioinformatics 22:768–770
Lanfear R, Calcott B, Ho SY, Guindon S (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol 29:1695–1701
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Loureiro M (2004) Sistemática y biogeografía de los peces anuales de la subtribu Cynolebiatina (Cyprinodontiformes: Rivulidae: Cynolebiatinae). PhD thesis, Montevideo
Loureiro M, Borthagaray A, Hernández D, Duarte A, Pinelli V, Arim M (2015) Austrolebias in space: scaling from ponds to biogeographical regions. In: Berois N, García G, de Sá RO (eds) Annual fishes: life history strategy, diversity, and evolution, 1st edn. Press Taylor & Francis group, Boca Raton, pp 111–132
Loureiro M, de Sá RO (2015) Diversity of Aplocheiloidei. In: Berois N, García G, de Sá RO (ed) Annual fishes: life history strategy, diversity, and evolution, 1st edn. Press Taylor & Francis group, Boca Raton, pp 3–31
Montaña JR, Bossi J (1995) Geomorfología de los humedales de la cuenca de la Laguna Merín en el departamento de Rocha. UDELAR. Revista Facultad de Agronomía. Montevideo, Uruguay 2:1–32
Nei M (1987) Molecular evolutionary genetics. Columbia Univ. Press, New York
Nielsen DTB, Pillet D (2015) Austrolebias accorsii, a new annual fish (Cyprinodontiformes: Rivulidae: Cynolebiatinae) from the upper río Grande basin, Amazon basin. Bolivia Aqua 21:172–179
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Szoecs E, Wagner H (2017) Vegan: community ecology package. R Package Version 2:4–3
Palumbi S, Martin A, Romano S, McMillan WO, Stice L, Grabowski G (1991) The simple fool’s guide to PCR. University of Hawaii, Honolulu
Ponce de León JL, León G, Rodríguez R et al (2014) Phylogeography of Cuban Rivulus: evidence for allopatric speciation and secondary dispersal across a marine barrier. Mol Phylogenet Evol 79:404–414
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rambaut A, Drummond AJ (2009) FigTree, v1.4.3. http://tree.bio.ed.ac.uk/software/figtree/. Accessed 18 July 2017
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Tracer v1.7. http://tree.bio.ed.ac.uk/software/tracer/. Accessed 6 July 2016
Reis RE, Lucena ZMS, Lucena CAS, Malabarba LR 2003. Peixes. In: Fontana CS, Bencke GA, Reis RE (ed) Livro vermelho da fauna ameaçada de extinção no Rio Grande do Sul, Edipucrs, Porto Alegre, pp 632
Rohlf FJ TpsDig Version 2.26 (2016) Department of ecology and evolution, stony brook 565 University, New York
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:1–4
Sambrook J, Fritschi EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York, p 1120
Staden R (1996) The Staden sequence analysis package. Mol Biotechnol 5:233–241
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Tajima F (1983) Evolutionary relationship of DNA sequences in finite populations. Genetics 105:437–460
Tajima F (1989) Statistical methods to test for nucleotide mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Templeton AR (2006) Population genetics and microevolutionary theory. Hoboken, New Jersey: John Wiley & Sons. In: Inc
Tomazelli LJ, Villwock JA (2000) O Cenozóico no Rio Grande do Sul: geologia da Planície Costeira. In: Holz M, De Ros LF (ed) Geologia do Rio Grande do Sul, CIGO/UFRGS, Porto Alegre, pp 375–406
Tomazelli LJ, Villwock JA (2005) Mapeamento Geológico de Planícies Costeiras: o Exemplo da Costa do Rio Grande do Sul. Gravel 3:109–115
Villwock JA, Tomazelli LJ (2007) Planície Costeira do Rio Grande do Sul: gênese e paisagem atual. In: Becker FG, Ramos RA, Moura LA (ed) Biodiversidade. Regiões da Lagoa do Casamento e dos Butiazais de Tapes, planície costeira do Rio Grande do Sul, Ministério do Meio Ambiente/SBF, pp 388
Volcan MV, Lanés LEK, Gonçalves AC, Guadagnin DL (2015) Annual fishes (Rivulidae) from Southern Brazil: a broad-scale assessment of their diversity and conservation. In: Berois N, García G, de Sá RO (ed) Annual fishes: life history strategy, diversity, and evolution, 1st edn. Press Taylor & Francis group, Boca Raton, pp 185–203
Whitlock MC (2000) Fixation of new alleles and the extinction of small populations: drift load, beneficial alleles, and sexual selection. Evolution 54:1855–1861
Wolf C, Rentsch J, Hübner P (1999) PCR-RFLP analysis of mitochondrial DNA: a reliable method for species identification. J Agr Food Chem 47:1350–1355
Acknowledgements
This study was funded by Fundação Grupo Boticário de Proteção à Natureza as part of the “Peixes Anuais do Pampa” and the “Padrões micro e macroevolutivos em peixes anuais de Cynopoecilus e Austrolebias (Cyprinodontiformes: Rivulidae) ao longo do Sistema de Drenagens Patos-Mirim: um enfoque comparativo com aplicações para a conservação – 1090_20171” projects. We thank CAPES and CNPq for providing fellowships to DKG and CB, respectively. All the collections were authorized by the Brazilian Ministério do Meio Ambiente (MMA), in the form of the Sistema de Autorização e Informação em Biodiversidade (SISBIO) (process number 55651-1). This study was also approved by the Ethics Comittee on Animal Use of the Universidade Federal do Rio Grande (process number 23116.008163/2015-23).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 1141 kb)
Rights and permissions
About this article
Cite this article
Garcez, D.K., Barbosa, C., Loureiro, M. et al. Phylogeography of the critically endangered neotropical annual fish, Austrolebias wolterstorffi (Cyprinodontiformes: Aplocheilidae): genetic and morphometric evidence of a new species complex. Environ Biol Fish 101, 1503–1515 (2018). https://doi.org/10.1007/s10641-018-0795-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-018-0795-2