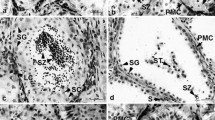
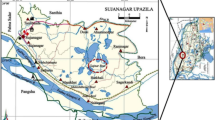
Abstract
Temperature and seasonal rainfall along with other environmental variables are important in regulating the reproductive cycles in teleost fishes. Certain environmental variables may act as cues for reproduction and changes in these may affect seasonality and success of reproduction, as fishes are known to integrate their physiological functions with environmental cycles. Wetlands are sensitive to climate change due to their shallow and confined nature. Since wetlands are important spawning and nursery grounds for many fishes, changes in the environmental variables may have direct consequences for the spawning and survival of fish. In the present study, we have assessed climatic and water chemistry variables capable of influencing seasonality in environmental variables as well as gonadal maturation of spotted snakehead Channa punctata, to predict threshold values of Gonado Somatic Index in females and a favourable range of identified climatic and water chemistry variables for breeding success. Among the climatic and water chemistry variables studied, seasonal variation in rainfall was found to have the most profound effect on gonadal maturation and breeding in C. punctata, followed by water temperature. The favourable range of rainfall obtained varied between 800 mm to 1400 mm, corresponding to the water temperature range between 29 °C and 31 °C. An overall significant warming trend with a reduction in total rainfall has been observed with changes in seasonal trends in temperature and rainfall in the study area. The rainfall being the major climatic factors influencing water chemistry in the wetlands during the spawning season, changes in rainfall pattern may influence breeding periodicity of C. punctata in wetlands in climate change scenario.






Similar content being viewed by others
References
Alvarado MV, Carrillo M, Felip A (2015) Melatonin-induced changes in kiss/gnrh gene expression patterns in the brain of male sea bass during spermatogenesis. Comparative Biochemistry and Physiology A 185:69–79. https://doi.org/10.1016/j.cbpa.2015.03.010
APHA (2012) Standard methods for examination of water and waste water, 22nd edn. American Public Health Association, Washington, DC
Baggerman B (1980) Photoperiod and endogenous control of the annual reproductive cycle in teleost fishes. In: Ali MA (ed) Environmental physiology of fishes. Plenum, New York, pp 533–567. https://doi.org/10.1007/978-1-4899-3659-2_21
Basak R, Roy A, Rai U (2016) Seasonality of reproduction in male spotted murrel Channa punctatus: correlation of environmental variables and plasma sex steroids with histological changes in testis. Fish Physiol Biochem 42:1249–1258
Bauer S, Nolet BA, Giske J, Chapman JW, Akesson S, Hedenström A et al (2011) Cues and decision rules in animal migration. In: Milner-Gulland EJ, Fryxell JM, Sinclair ARE (eds) Animal migration – a synthesis. Oxford University Press, Oxford, pp 68–87. https://doi.org/10.1093/acprof:oso/9780199568994.003.0006
Bhattacharyya S, Maitra S (2006) Environmental correlate of the testicular events in a major carp Catla catla in an annual reproductive cycle. Biol Rhythm Res 37(2):87–110. https://doi.org/10.1080/09291010500124605
Bhuiyan AS, Rahman K (1984) Fecundity of snake-head-fish Channa punctatus. J Asiatic Soc Bangladesh (Sci) 10:75–81
Bogard JR, Thilsted SH, Marks GC, Wahab MA, Hossain MAR, Jakobsen J, Stangoulis J (2015) Nutrient composition of important fish species in Bangladesh and potential contribution to recommended nutrient intakes. J Food Compos Anal 42:120–133. https://doi.org/10.1016/j.jfca.2015.03.002
Bruton M (1979) The breeding biology and early development of Clarias gariepinus (Pisces: Clariidae) in Lake Sibaya, South Africa, with a review of breeding in species of the subgenus Clarias (Clarias). Transactions of the Zoological Society of London 35:1–45
Chaudhry S (2010) Channa punctata. The IUCN red list of threatened species 2010: e.T166437A6209123. https://doi.org/10.2305/IUCN.UK.2010-4.RLTS.T166437A6209123
Chellappa S, Câmara MR, Chellappa NT, Beveridge MCM, Huntingford FA (2003) Reproductive ecology of a Neotropical cichlid fish Cichla monoculus (Osteichthyes, Cichlidae). Braz J Biol 63(1):17–26. https://doi.org/10.1590/S1519-69842003000100004
Clark FN (1934) Maturity of the California sardine (Sardina cacrulea) determined by ova diameter measurements. Calif Dill Fish Game 42:1–49
Cognato Diego de P, Bernhardt FC (2006) Reproductive biology of a population of Gymnotus aff. carapo (Teleostei: Gymnotidae) from southern Brazil. Neotropical Ichthyology 4(3):339–348
Combes (2003) Protecting freshwater ecosystems in the face of global climate change. In: Hansen LJ, Biringer JL, Hoffman JR (eds) A user’s manual for building resistance and resilience to climate change in natural systems. WWF, Germany, pp 177–216
Cooke SJ (2014) Conservation physiology today and tomorrow. Conservation. Physiology 2:1–3
Cooke SJ, Hultine KR, Rummer JL, Franklin CE (2017) Reflections and progress in conservation physiology. Conservation Physiology 5(1). https://doi.org/10.1093/conphys/cow071
Das MK, Srivastava PK, Dey S, Mandal ML, Sahu SK (2011) Impact of draught on hatchery fish seed production in West Bengal- a case study. J Inland Fish Soc India 43(1):25–32
Das MK, Srivastava PK, Dey S, Rej A (2012) Impact of temperature and rainfall alterations on spawning behaviour of Indian major carps and consequence on fishers’ income in Odisha. J Inland Fish Soc India 44(2):1–11
Das MK, Sharma AP, Sahu SK, Srivastava PK, Rej A (2013) Impacts and vulnerability of inland fisheries to climate change in the Ganga River system in India. Aquat Ecosyst Health Manag 16(4):415–424. https://doi.org/10.1080/14634988.2013.851585
Das MK, Srivastava PK, Rej A, Mandal ML, Sharma AP (2016) A framework for assessing vulnerability of inland fisheries to impacts of climate variability in India. Mitig Adapt Strateg Glob Chang 21(2):279–296. https://doi.org/10.1007/s11027-014-9599-7
Davies PR, Hanyu I, Furukawa K, Nomura M (1986) Effect of temperature and photoperiod on sexual maturation and spawning of the common carp. Induction of Spawning by Manipulating Photoperiod and Temperature Aquacult 52:137–144
de Vlaming VL (1974) Environmental and endocrine control of teleost reproduction. In: Schreck CB (ed) Control of sex in fishes. Virginia Polytechnic Institute and State University, Blacksburg, pp 13–83
Dey S, Srivastava PK, Maji S, Das MK, Mukhopadhyaya MK, Saha PK (2007) Impact of climate change on the breeding of Indian major carps in West Bengal. J Inland Fish Soc India 39(1):26–34
do Vale JD, Zuanon J, Magnusson WE (2014) The influence of rain in limnological characteristics of Viruá wetlands, Brazilian Amazon. Acta Limnologica Brasiliensia 26(3):254–267
Erwin KL (2009) Wetlands and global climate change: the role of wetland restoration in a changing world. Wetl Ecol Manag 17(1):71–84. https://doi.org/10.1007/s11273-008-9119-1
Falcón J, Migaud M, Muñoz-Cueto JA, Carrillo M (2010) Current knowledge on the melatonin system in teleost fish. Gen Comp Endocrinol 165(3):469–482. https://doi.org/10.1016/j.ygcen.2009.04.026
Fox J, Monette G (1992) Generalized collinearity diagnostics. J Am Stat Assoc 87(417):178–183. https://doi.org/10.1080/01621459.1992.10475190
Gam L, Leow C, Baie S (2006) Proteomic analysis of snakehead fish (Channa striata) muscle tissue. Malays. J Biochem Mol Biol 14:25–32
Graff L, Middleton J (2001) Wetlands and fish: catch the link. National Oceanic and Atmospheric Administration. National Marine Fisheries Services, Maryland
Graham CT, Harrod C (2009) Implications of climate change for the fishes of the British isles. J Fish Biol 74(6):1143–1205. https://doi.org/10.1111/j.1095-8649.2009.02180.x
Groemping U (2006) Relative importance for linear regression in R: the package relaimpo. J Stat Softw 17(1):1–27
Hartmann DL, Klein Tank AMG, Rusticucci M, Alexander LV, Brönnimann S, Charabi Y, Dentener FJ, Dlugokencky EJ, Easterling DR, Kaplan A, Soden BJ, Thorne PW, Wild M, Zhai PM (2013) Observations: atmosphere and surface. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK., Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis (Contributions of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge: 159–254
Hastie T, Tibshirani R (1986) Generalized additive models. Stat Sci 1(3):297–318. https://doi.org/10.1214/ss/1177013604
Hossain MA, Mian S, Akter M, Rabby AF, Marine SS, Rahman MA, Iqbal MM, Islam MJ, Hassan MM, Hossain MM (2015) Ovarian biology of spotted snakehead (Channa punctatus) from natural wetlands of Sylhet, Bangladesh. Ann Vet Anim Sci 2(3):64–76
Htun-Han M (1978) The reproductive biology of the dab Limanda limanda (L.) in the North Sea: seasonal changes in the ovary. J Fish Biol 13(3):351–359. https://doi.org/10.1111/j.1095-8649.1978.tb03443.x
Hulme PE (2005) Adapting to climate change: is there scope for ecological management in the face of a global threat? J Appl Ecol 42(5):784–794. https://doi.org/10.1111/j.1365-2664.2005.01082.x
IPCC (2014) Climate change 2014: impacts, adaptation and vulnerability. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL (eds) Part a:global and sectoral aspects (contribution of working group II to the fifth assessment report of the intergovernmental panel on climate change). Cambridge University Press, Cambridge
Jollife IT (1972) Discarding variables in a principal component analysis I: arti®cial data. Appl Stat 21(2):160–173. https://doi.org/10.2307/2346488
Jonsson B, Jonsson N (2009) Migratory timing, marine survival and growth of anadromous brown trout in the river Imsa, Norway. J Fish Biol 74(3):621–638. https://doi.org/10.1111/j.1095-8649.2008.02152.x
Junk WJ (1997) The central Amazon floodplain: ecology of a pulsing system. Berlin: springer Verlag. Ecological Studies 525p
Kaushal SS, Likens GE, Jaworski NA, Pace ML, Sides AM, Seekell D, Kenneth TB, David HS, Wingate RL (2010) Rising stream and river temperatures in the United States. Front Ecol Environ 8(9):461–466. https://doi.org/10.1890/090037
Khan H (1924) Observations on the breeding habits of some freshwater fishes in the Punjab. J Bombay Nat Hist Soc 29:958–962
Kramer DL (1978) Reproductive seasonality in the fishes of a tropical stream. Ecology 59(5):976–985. https://doi.org/10.2307/1938549
Lam T (1983) Environmental influences on gonadal activity in fish. In: Hoar WS, Randall DJ, Donaldson EM (eds) fish physiology, Vol IXB. Academic, New York, pp 65–116
Lowe-McConnell RH (1987) Ecological studies in tropical fish communities. Cambridge university press, England. https://doi.org/10.1017/CBO9780511721892
Lynch AJ, Myers BJE, Chu C, Eby LA, Falke JA, Kovach RP, Krabbenhoft TJ, Kwak TJ, Lyons J, Paukert CP, Whitney JE (2016) Climate change effects on north American inland fish populations and assemblages. Fisheries 41(7):346–361. https://doi.org/10.1080/03632415.2016.1186016
Lyons J, Andrew LR, Paul WR, Thomas EB, Bradley TE, Jared TM, Tammie JP, Peter BM (2015) Trends in the reproductive phenology of two Great Lakes fishes. Trans Am Fish Soc 144(6):1263–1274. https://doi.org/10.1080/00028487.2015.1082502
McNamara JM, Barta Z, Klaassen M, Bauer S (2011) Cues and the optimal timing of activities under environmental changes. Ecol Lett 14(12):1183–1190. https://doi.org/10.1111/j.1461-0248.2011.01686.x
Molur S, Walker S (1998) Report of the workshop on “conservation assessment and management plan for freshwater fishes of India”. Zoo outreach organisation (Conservationbreeding specialist group), Coimbatore
Mookerjee HK (1945) Identification of eggs of common freshwater fishes of Bengal. Sci Cult 11:48
Mookerjee HK, Mazumdar SR, Das Gupta BN (1944) Observation on breeding ground and spawning habits of certain Indian major carps in Midnapore district, Bengal with suggestions for their breeding, collection of eggs and rearing of fry. Journal of Department of Science 1(4):81–91
Nikolsky GV (1963) The ecology of fishes. Academic, London, 233p
Pankhurst NW, King HR (2010) Temperature and salmonid reproduction: implications for aquaculture. J Fish Biol 76(1):69–85. https://doi.org/10.1111/j.1095-8649.2009.02484.x
Pankhurst N, Porter M (2003) Cold and dark or warm and light: variations on the theme of environmental control of reproduction. Fish Physiol Biochem 28(1-4):385–389. https://doi.org/10.1023/B:FISH.0000030602.51939.50
Patrick H, de Souza TG, Moreira RG, Nakaghi LSO and Batlouni SR (2012) Gonadal steroids levels and vitellogenesis in the formation of oocytes in Prochilodus lineatus (Valenciennes) (Teleostei: Characiformes. Neotropical Ichthyology 10(3):601–612
Perkin JS, Shattuck ZR, Bonner TH (2012) Life history aspects of a relict ironcolor shiner Notropis chalybaeus population in a novel spring environment. Am Midl Nat 167(1):111–126. https://doi.org/10.1674/0003-0031-167.1.111
Prasad L, Dwivedi AK, Dubey VK, Serajuddin M (2011) Reproductive biology of freshwater murrel, Channa punctatus (Bloch, 1793) from river Varuna (a tributary of Ganga River) in India. J Ecophysiol Occup Hlth 11:69–80
R Core Team (2017) R: a language and environment for statistical computing. In: RFoundation for statistical computing. URL, Vienna https://www.R-project.org/
Raj SB (1916) Notes on the freshwater fish of madras. Rec Indian Mus XII, part VI:249–294
Ramsar Convention Secretariat (2013) The Ramsar convention manual: a guide to the convention on wetlands (Ramsar, Iran, 1971), 6th edn. Ramsar Convention Secretariat, Gland
Reddy PB (1979) Maturity and spawning in the murrel, Channa punctata (block 1973) (Pisces, Teleostei, Channidae) from Guntur, Andhra Pradesh. Proc Indian Natl Sci Acad 45:543–553
Ruiz Navarro A, Gillingham PK, Britton JR (2016) Shifts in the climate space of temperate cyprinid fishes due to climate change are coupled with altered body sizes and growth rates. Glob Chang Biol 22(9):3221–3232. https://doi.org/10.1111/gcb.13230
Servili A, Herrera-Pérez P, Rendón MC, Muñoz-Cueto JA (2013) Melatonin inhibits GnRH-1, GnRH-3 and GnRH receptor expression in the brain of the European sea bass, Dicentrarchus labrax. Int J Mol Sci 14(4):7603–7616. https://doi.org/10.3390/ijms14047603
Shrestha TK (1990) Resource ecology of the Himalayan waters. Tribhuvan University, Kathmandu, Curriculum Development Centre
Singh N, Gupta PK (2014) Reproductive biology of eastern mosquito fish Gambusia holbrooki (Girard Poeciliiadae) in a sub-tropical lake, Lake Nainital (India). Int J Curr Microbiol Appl Sci 3:19–31
Singh R, Chaturvedi SK, Abhinav (2010) Effect of photoperiod and temperature on testicular regression in Channa punctatus. J Environ Biol 31:307–310
Sundararaj BI (1978) Environmental regulation of annual reproductive cycles in the catfish, Heteropneustes fossilis. In: Assenmacher I, Famer DS (eds) Environmental endocrinology. Springer-Verlag, Berlin, 267p. https://doi.org/10.1007/978-3-642-66981-1_5
Sundararaj BI, Sehgal A (1970) Effects of a long or an increasing photoperiod on the initiation of ovarian recrudescence during the preparatory period in the catfish, Heteropneustes fossilis (Bloch). Biol Reprod 2(3):413–424. https://doi.org/10.1095/biolreprod2.3.413
Takemura A, Takeuchi Y, Ikegami T, Sung-Pyo H, Soliman V, Felix A, de Jesus-Ayson G (2015) Environmental control of annual reproductive cycle and spawning rhythmicity of Spinefoots. Endang Sri Susilo5. Kuroshio Science 9(1):31–38
Tandon KK (1963) Biology of Channa punctatus (Bloch.) and Glossogobius giuris (ham.) Res Bull Punjab Univ 13:257–262
Vass KK, Das MK, Srivastava PK, Dey S (2009) Assessing the impact of climate change on inland fisheries in river ganga and its plains in India. Aquat Ecosyst Health Manag 12(2):138–151. https://doi.org/10.1080/14634980902908746
Visser ME, Caro SP, van Oers K, Schaper SV, Helm B (2010) Phenology, seasonal timing and circannual rhythms: towards a unified framework. Philos Trans R Soc Lond 365(1555):3113–3127. https://doi.org/10.1098/rstb.2010.0111
Whitney JE, Chokhachy RA, Bunnell DB, Caldwell CA, Cooke SJ, Eliason EJ, Rogers M, Lynch AJ, Paukert CP (2016) Physiological basis of climate change impacts on north American inland fishes. Fisheries 41(7):332–345. https://doi.org/10.1080/03632415.2016.1186656
Wiebe J (1968) The effects of temperature and daylength on the reproductive physiology of the viviparous sea perch, Cymatogaster aggregata gibbons. Can J Zool 46(6):1207–1219. https://doi.org/10.1139/z68-171
Acknowledgements
Authors are thankful to the Director, ICAR-Central Inland Fisheries Research Institute, Barrackpore for his endless support. The financial support in the project National Innovations in Climate Resilient Agriculture (NICRA) is also gratefully acknowledged. We would also like to acknowledge the services rendered by fishermen of Bhomra Fisheries Co-operative Society, District Nadia, West Bengal (India). The authors would also like to acknowledge the reviewers for their critical and constructive suggestions for improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karnatak, G., Sarkar, U.K., Naskar, M. et al. Understanding the role of climatic and environmental variables in gonadal maturation and spawning periodicity of spotted snakehead, Channa punctata (Bloch, 1793) in a tropical floodplain wetland, India. Environ Biol Fish 101, 595–607 (2018). https://doi.org/10.1007/s10641-018-0722-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-018-0722-6